The intersection of physics and biology has yielded profound insights into understanding complex systems that govern cellular behavior. Research conducted by scientists at São Paulo State University (UNESP) in Brazil taps into classical mixture theory, originally utilized in physics, to delve into the dynamics of protein compartmentalization in cells. Their findings, as published in the journal Heliyon, suggest a cellular phase analogous to the magnetic Griffiths phase, demonstrating the significance of interdisciplinary approaches to untangle intricate biological phenomena.
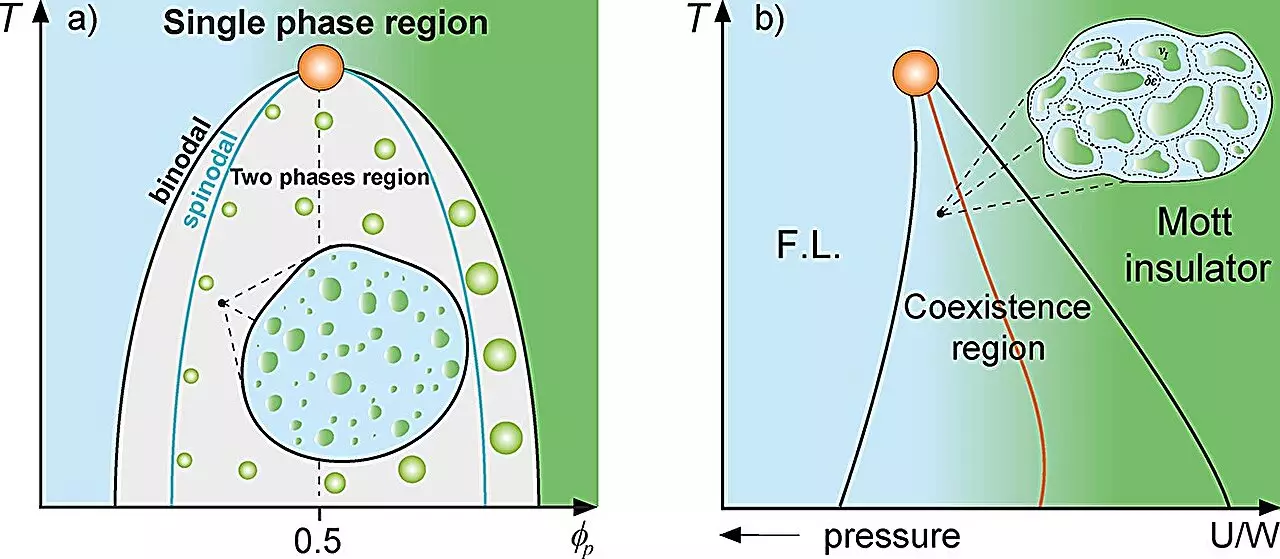
To appreciate the implications of this research, one must first comprehend the concept of the Griffiths phase in magnetism. Within a magnetic framework, regions of differing magnetic properties—magnetized or un-magnetized—occur in a paramagnetic or ferromagnetic matrix. These ‘rare regions’ disrupt the homogeneity of the system, leading to reduced dynamics. Translating this idea to cell biology, researchers identified protein droplets that emerge in response to certain cellular conditions as analogous to these rare regions. This parallels previous investigations into the electronic Griffiths-like phase, particularly at transitions such as the Mott metal-insulator crossover.
The study reveals that protein production within cells can provoke liquid-liquid phase separation, which leads to the compartmentalization of proteins into distinct droplets. By employing thermodynamic models—including the Grüneisen parameter and Flory-Huggins model—the researchers examined how cellular dynamics are diminished near the binodal line representing phase separation. This threshold might represent a critical point influencing cellular organization and functional efficiency, echoing findings in physical systems that showcase phase separation phenomena.
As cells manage their environment with proteins that exhibit varying concentrations and interactions, the concept of a Griffiths-like cellular phase becomes increasingly relevant. Mariano de Souza, the principal investigator, asserts that understanding these compartments is crucial for unpacking the ways in which cellular dynamics are altered in different biological contexts, potentially impacting gene expression and cellular responses.
The researchers extend the implications of their findings to the origins of life, drawing on the classic theories proposed by Russian biochemist Aleksandr Oparin in the 1930s. Oparin postulated that coacervates—aggregated organic molecules in aqueous environments—played a vital role in the early stages of life evolution. The researchers suggest that only those droplets with slower dynamics, akin to the Griffiths-like cellular phase described, were capable of surviving and evolving. This observation ties the compartmentalization of proteins not merely to cell functionality but also to the fundamental processes that may have led to life itself.
In a broader biological context, the study hints at the importance of homochirality—the predominance of a single molecular chirality—in evolutionary biology. This phenomenon has far-reaching implications for understanding how biological systems have developed and optimized their functions over time.
The research also unveils important connections between liquid-liquid phase separation and clinical phenomena. Marcos Minicucci, a co-author of the study, emphasizes that aberrant protein compartmentalization may contribute to diseases such as cancer and neurodegenerative conditions. For example, proteins related to tumorigenesis might be partitioned in a manner that disrupts normal cellular function. This highlights the duality of protein droplet formation, implying that while it may offer benefits under certain conditions, it can also lead to detrimental effects in pathological states.
Additionally, the findings resonate with ongoing therapeutic research, indicating that manipulating the dynamics of these protein droplets might serve as a novel intervention strategy. Various diseases, from cataracts to COVID-19, further illustrate the relevance of phase separation in understanding pathology and potential treatment modalities.
The research undertaken by the UNESP team not only advances the understanding of protein dynamics but also underscores the value of incorporating principles from physics into biological research. By recognizing the parallels between physical and biological systems, scientists can uncover novel insights that drive innovation in both fields. The concepts of the Griffiths-like cellular phase and liquid-liquid phase separation could serve as pivotal frameworks for exploring not only cell biology but also the genesis of life and the complexities involved in various diseases. This work highlights a promising avenue for future interdisciplinary research, with the potential to unravel further mysteries of cellular organization and function.