A pioneering study from a team led by Professor Jaeheung Cho at UNIST sheds light on the intricate interaction between cobalt(III)-based metal complexes and nitrile compounds. Published in the esteemed *Journal of the American Chemical Society*, this research not only uncovers the underlying mechanisms of nitrile activation but also opens new avenues for the development of pharmaceuticals. The investigation into these biomimetic compounds reveals vital insights into how metal spin states can significantly affect reaction dynamics, offering promising implications for medical chemistry.
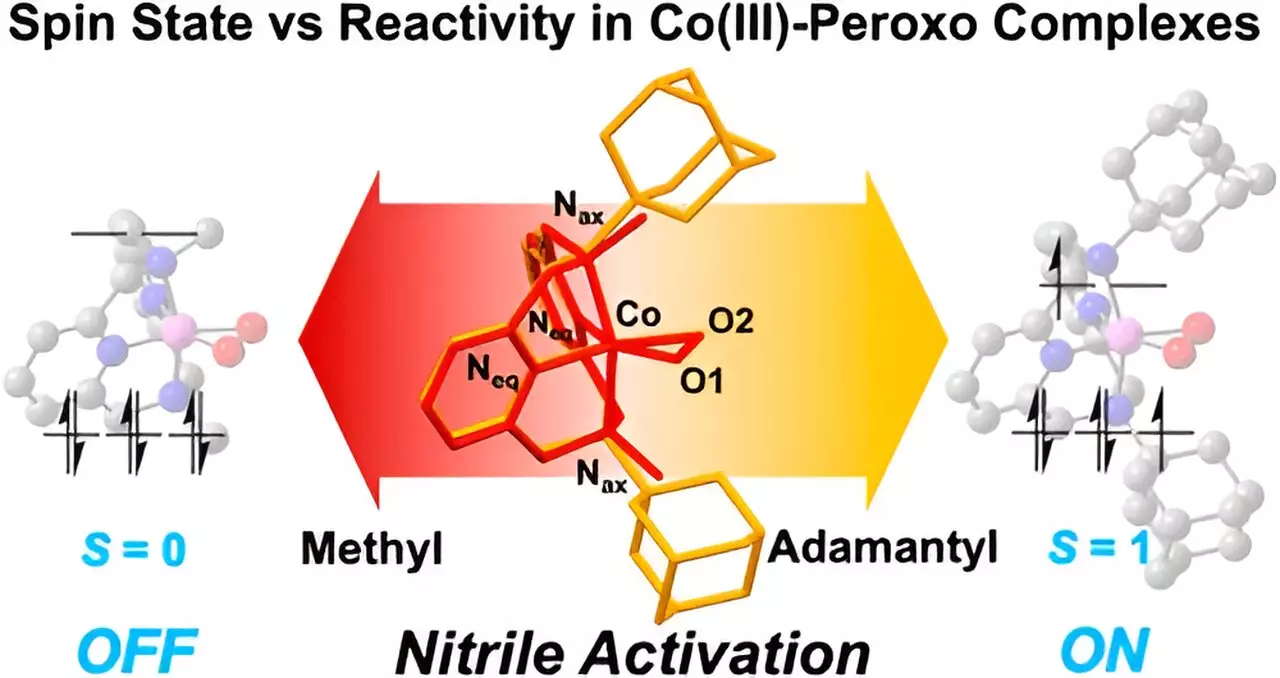
At the heart of this study is the exploration of how subtle changes in metal properties can dramatically influence the speed and outcomes of chemical reactions. For instance, the research team’s focus on the “Macrocyclic Pyridinophane System” allowed for the modification of cobalt compound structures, providing a platform to analyze how nitriles interact with these complexes. Notably, the findings emphasize that the metal’s spin states play a crucial role in determining reactivity. By illustrating that variations in the size of functional groups can alter spin states, the researchers successfully showcased distinct reactivity profiles in cobalt complexes.
A key aspect of the research involved examining how the size of functional groups affects the reactivity of cobalt(III) complexes. The team’s experimentation revealed that larger adamantyl groups led to a greater activation of nitriles, while smaller groups, specifically methyl groups, exhibited negligible reactivity. This stark contrast underscores the importance of structural characteristics in facilitating or hindering chemical reactions. The results effectively demonstrate that the three-dimensional structure of ligands critically influences the properties and efficacy of metal complexes in reaction pathways.
Nitriles are prevalent in a variety of applications, including pharmaceuticals and pesticides, yet their reactivity has often posed challenges. The UNIST team’s findings address these challenges head-on by demonstrating that cobalt(III)-peroxo species can successfully react with nitriles at room temperature, catalyzing the formation of new compounds with potential anticancer properties. As first author Seonghan Kim notes, the successful synthesis of cobalt(III)-peroxo species with different spin states validates the connection between ligand configuration and nitrile reactivity.
The implications of this research extend far beyond mere academic interest; they mark a significant step forward in the quest for new drug development. The ability to manipulate metal complexes to enhance reactivity could lead to novel compounds with therapeutic applications, notably in cancer treatment. The research highlights a promising strategy to navigate the intricate landscape of chemical reactivity, paving the way for future innovations in medicinal chemistry. In the pursuit of more effective pharmaceuticals, the findings of this study offer a valuable roadmap for scientists looking to harness the unique properties of cobalt(III) complexes.