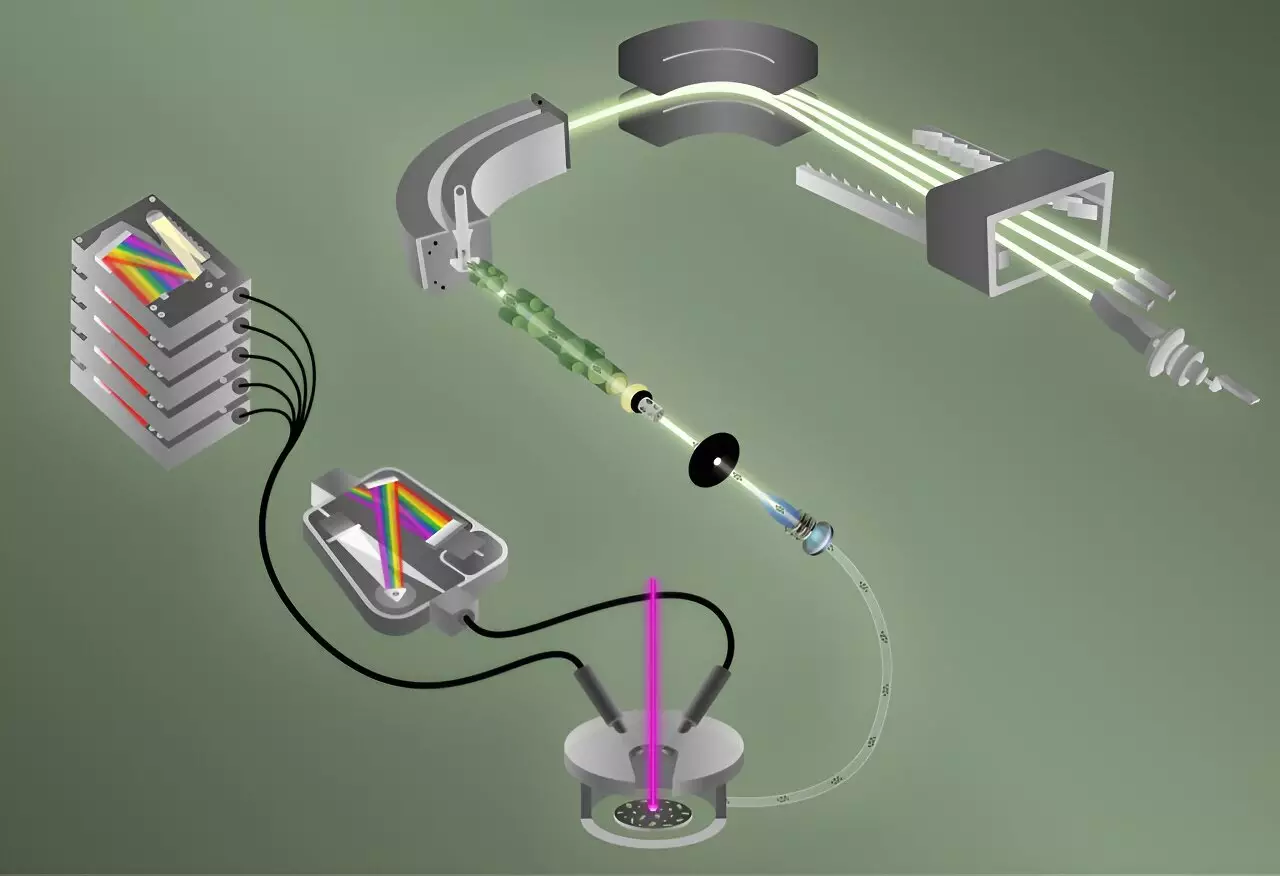
In the landscape of nuclear material analysis, the ability to accurately characterize elemental and isotopic compositions is vital for both scientific inquiry and national security. Researchers at the Oak Ridge National Laboratory (ORNL), part of the U.S. Department of Energy, have taken a significant step forward with the development of a pioneering technique that enables simultaneous detection of fluorine and uranium isotopes within a single particle. This innovative approach, published in the Journal of the American Chemical Society, represents a remarkable advancement in the realm of analytical chemistry. By combining laser-induced breakdown spectroscopy (LIBS) with laser ablation multi-collector inductively coupled plasma mass spectrometry (ICP-MS), the ORNL team has successfully enhanced the efficiency and speed at which single particles can be analyzed.
Fluorine and uranium are not just important from a scientific perspective; they hold significant implications for nuclear nonproliferation. The presence of fluorine is critical for the conversion of uranium into a form that can be enriched for use in nuclear processes, making its detection alongside uranium pivotal for inspectors from the International Atomic Energy Agency (IAEA). The ability to assess both elements in conjunction can provide insights into the intended use and origin of nuclear materials, arguably enhancing our understanding of global nuclear security dynamics. The lead researcher, Benjamin Manard, emphasizes that this unprecedented capability allows for rapid assessment, a marked improvement over traditional methods that often require considerable time to establish isotopic ratios for particles.
The analysis commenced with the utilization of LIBS, which boasts the ability to identify and quantify elements like fluorine by vaporizing a sample and forming a plasma cloud. This cloud emits light as it cools, which is then spectrally analyzed. In a metaphor likening the process to fireworks, Manard highlights how different elements radiate distinct wavelengths of light, thus facilitating their identification.
The second technique employed, ICP-MS, complements LIBS by scrutinizing the isotopes of uranium present in the particle. The unique conditions in the plasma created by the ICP-MS—exceeding 8,000 kelvin—allow for the effective formation of positive ions essential for mass spectrometry. This duality is imperative since fluorine’s electronegativity poses challenges for traditional ICP-MS measurement, which typically targets positive ions. The researchers ingeniously overcame this hurdle, successfully integrating LIBS with ICP-MS for their experiments.
Implications Beyond Nuclear Nonproliferation
While the primary application of this technique pertains to nuclear security, its implications extend far beyond that frontier. The ability to characterize complex materials with such precision could find application in a diverse range of fields. For instance, understanding fluorine distribution in biological samples, such as shark teeth, may unlock information about environmental changes over time. This interdisciplinary approach not only demonstrates the flexibility and adaptability of the technique but also exemplifies a novel intersection of nuclear science with environmental and biological research.
The potential applications also include advancements in next-generation battery manufacturing and the design of fuels for advanced nuclear reactors, underscoring the relevance of this research across internal sectors.
The success of this endeavor is a testament to the power of interdisciplinary collaboration. The project engaged a diverse team at ORNL, drawing expertise from various fields to bring these techniques to fruition. From specialists in mass spectrometry to researchers focusing on traditional spectroscopic methods, collaboration was crucial for overcoming technical challenges and refining experiments. This synergy not only led to significant advancements in the analysis techniques themselves but also positions ORNL as a leader in the field of nuclear materials characterization.
Future Directions and Enhancements
Looking forward, Manard and his team are keen on expanding the capabilities of their integrated technique. They envision applying these methods to better understand other challenging compounds relevant to nuclear processes, such as chlorine, which shares similar properties with fluorine. As their research progresses, the ambition to push the boundaries of particle analysis—enabling the characterization of thousands of particles in a single day—remains a guiding principle.
The dual technique established at Oak Ridge National Laboratory represents a significant stride in nuclear material analysis, merging sensitivity with speed and portability. Its implications reach far beyond mere nuclear nonproliferation monitoring, offering insights into various scientific domains and emphasizing the importance of collaborative research in fostering innovation.