A significant advancement has been made by chemists from the National University of Singapore (NUS) that promises to reshape the methodologies in organic chemistry. Their groundbreaking research revolves around an iron-catalyzed approach that facilitates the formation of trisubstituted Z-alkenes from allenes, marking a leap toward sustainable chemical synthesis. Published in the renowned journal, Nature Synthesis, this research highlights the potential for more eco-friendly practices in creating complex organic compounds which are vital for drug development and other applications.
Trisubstituted alkenes serve as key components in biologically active molecules and are crucial substrates in a variety of stereospecific reactions. However, synthesizing Z-isomers—structurally distinct due to their molecular configuration—poses a notable challenge, primarily because they tend to be less thermodynamically stable than their E-isomer variants. Traditional methods often fall short, as they fail to achieve the necessary selectivity. The search for an efficient catalytic technique that could enable the production of Z-isomers was fraught with difficulty, thus underscoring the importance of the NUS team’s findings.
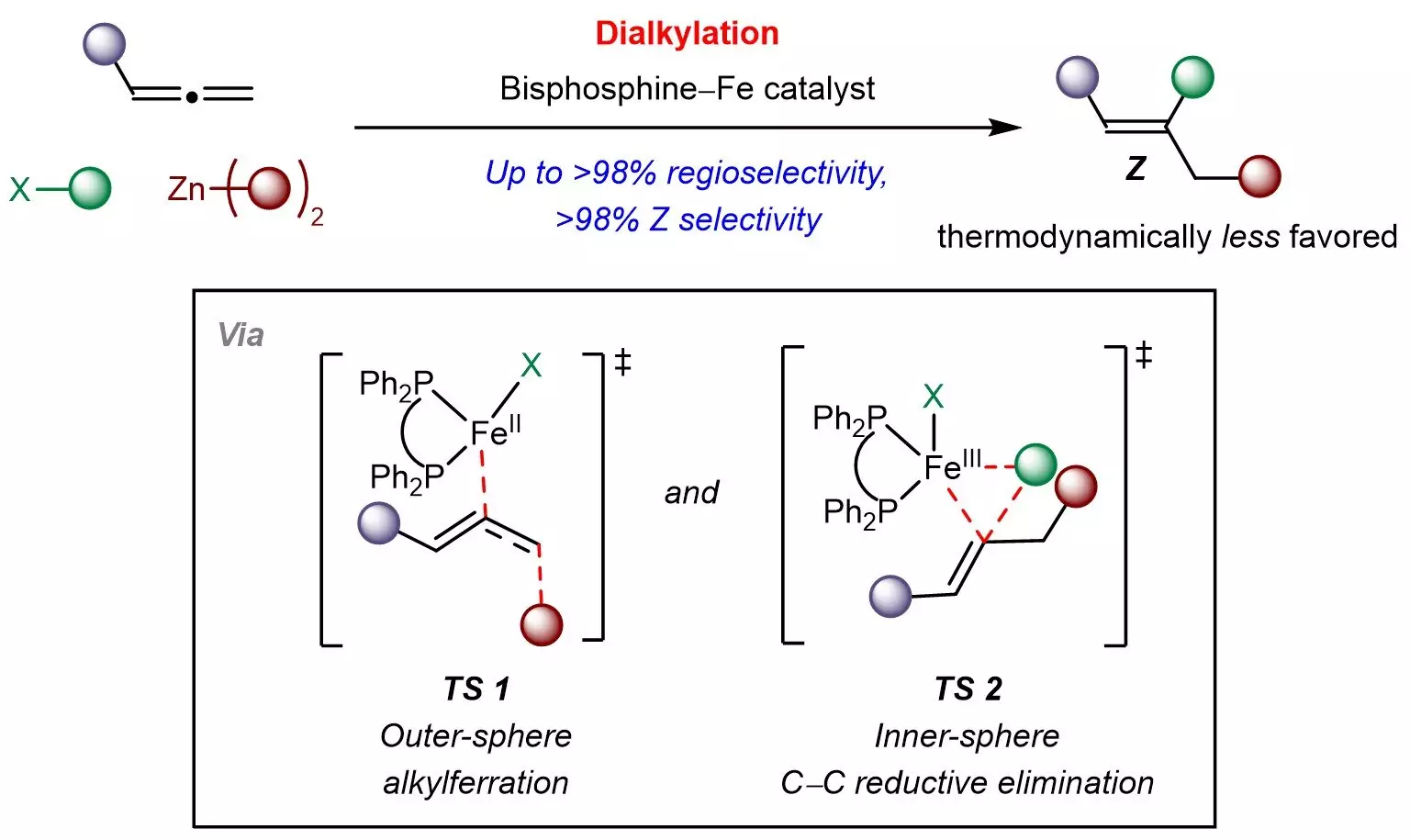
At the heart of this innovative research is a versatile iron catalyst, developed by a team led by Associate Professor Koh Ming Joo. This new method employs a biphophine–iron catalyst and integrates it with organohalides and organozinc reagents—a clever combination that results in an effective multicomponent strategy. By successfully controlling the site of reaction and achieving a high degree of selectivity for the Z-isomers, it not only addresses a prior gap in chemical synthesis but does so with a sustainable approach, capitalizing on iron’s abundance and low toxicity.
An exciting application of this synthetic method was illustrated in the preparation of a glucosylceramide synthase inhibitor, where the trisubstituted Z-alkene configuration was essential for its biological effectiveness. This tangible advantage underscores the method’s capability to facilitate the production of complex molecules that are otherwise challenging to synthesize. Collaborations with institutions like The Chinese University of Hong Kong and A*STAR further emphasize the far-reaching implications of this research across the chemical and pharmaceutical landscapes.
Moreover, the research elucidates a novel mechanism characterized by outer-sphere radical-mediated processes followed by inner-sphere carbon-carbon bond formation. This mechanistic insight is a pivotal contribution to the understanding of kinetically directed reactions, potentially inspiring researchers to explore other multicomponent transformations using readily available raw materials.
The NUS team’s innovative work in iron-catalyzed reactions not only enriches the existing literature on alkene synthesis but can also catalyze advancements in various industrial applications. With an emphasis on sustainability, this approach represents a significant step forward in the quest for greener chemistry. As Prof. Koh aptly noted, this method facilitates deeper exploration of difficult-to-access hydrocarbon compounds, promising exciting possibilities in drug discovery and beyond.