Hydrogen gas is increasingly being recognized as a keystone in the quest for a sustainable and environmentally friendly energy landscape. Not only is hydrogen the most abundant element in the universe, but its carbon-free combustion makes it an attractive candidate for various energy applications. However, its inherent challenges often stem from the fact that hydrogen primarily exists in bound states, such as ammonia and other hydrogen-rich compounds. Among these carriers, ammonia (NH₃) emerges as a particularly promising option due to its high hydrogen content, making up approximately 17.6% of its mass, as well as its relative abundance, ease of storage, and transportation.
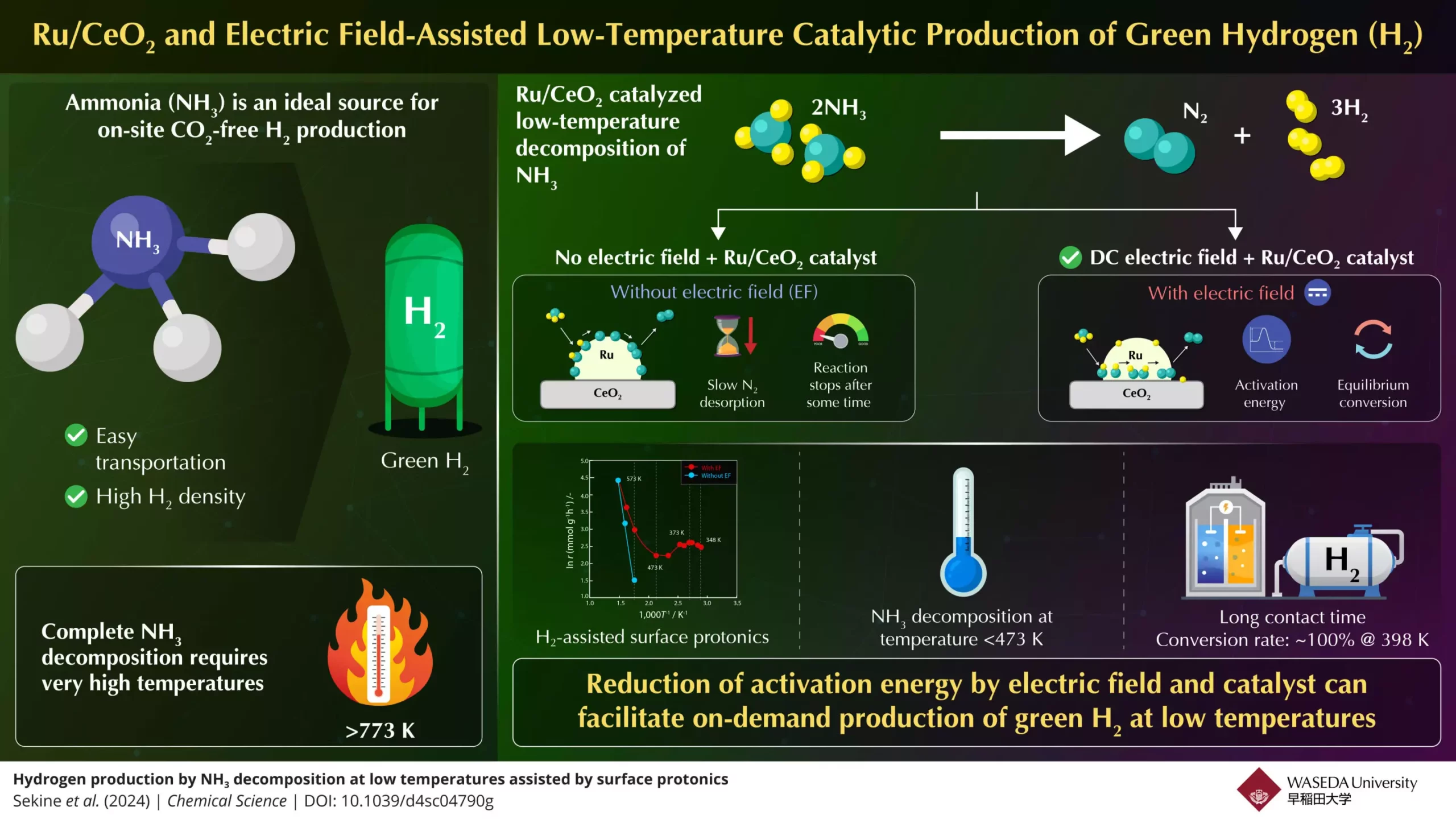
Despite the advantages that ammonia offers, its widespread use as a reliable source of green hydrogen faces significant hurdles. A notable roadblock is the requirement for high temperatures, often exceeding 773 K, to facilitate the decomposition of ammonia into hydrogen and nitrogen. This limitation presents challenges for practical applications, including fuel cell technologies and internal combustion engines, where efficient and low-temperature hydrogen production is essential. Without effective strategies to lower the activation energy needed for this process, ammonia’s transition from a hydrogen carrier to a hydrogen source remains constrained.
In light of these challenges, a research team led by Professor Yasushi Sekine at Waseda University has recently made strides in developing a compact process designed to operate effectively at lower temperatures. Collaborating with experts from Yanmar Holdings, the team aims to harness ammonia’s potential for on-demand hydrogen generation. Their groundbreaking work revolves around an innovative experimental setup that employs an electric field in conjunction with a highly efficient Ru/CeO₂ catalyst to achieve a faster rate of ammonia-to-hydrogen conversion.
The team’s approach addresses the two critical steps in the reaction sequence: the desorption of nitrogen and the dissociation of the N–H bonds. At lower temperatures, nitrogen desorption has been identified as the rate-limiting step, while high temperatures typically support the dissociation of the N–H bonds. Sekine and his associates recognized the need for a dual approach to tackle both challenges and enhance ammonia conversion efficiency.
Electric Field-Assisted Catalytic Reactions
The team employed electric field-assisted catalytic reactions as a solution. By applying a direct current (DC) electric field to the Ru/CeO₂ catalyst, they observed an increase in proton conduction on the catalyst surface, effectively lowering the activation energy needed for the reaction. The findings indicated that ammonia could be decomposed efficiently even at temperatures below 473 K. Remarkably, in an optimal scenario with sufficient contact time between the ammonia feed and the catalyst, the researchers achieved a 100% conversion rate at just 398 K.
The role of the electric field was pivotal in promoting surface protonics—essentially aiding in the hopping of protons across the catalyst surface. This enhancement in proton mobility directly correlated with the lowering of the apparent activation energies and increased rates of the ammonia conversion process.
Significance and Future Implications
The implications of this research are profound, as it suggests that green hydrogen can be produced sustainably from ammonia at relatively low temperatures, utilizing an irreversible pathway that facilitates high conversion rates. Not only does this contribute positively to the ongoing transition to cleaner energy sources, but it also opens the door for the practical implementation of ammonia as a hydrogen carrier in various industrial applications.
Professor Sekine highlights the broader significance of their findings, asserting that this method could facilitate the widespread adoption of clean alternative fuels. By simplifying the on-demand synthesis of CO₂-free hydrogen, they have paved the way for hydrogen fuel to become more accessible and practical for various applications, thereby contributing to global sustainability goals.
The research team at Waseda University has made a notable advancement in ammonia conversion technology, presenting a viable solution to one of the key hurdles in utilizing ammonia as a hydrogen source. As nations and industries grapple with the pressing need for cleaner energy solutions, such innovative approaches hold the promise of revolutionizing energy systems for a sustainable future.