In a significant advancement in the field of cancer research, scientists at the Department of Energy’s Oak Ridge National Laboratory have gained a deeper understanding of an enzyme known as serine hydroxymethyltransferase (SHMT). Through groundbreaking neutron experiments, they have elucidated the precise atomic-scale interactions of SHMT—an essential enzyme involved in cellular metabolism and proliferation. This could potentially open new avenues for designing effective inhibitors to combat aggressive forms of cancer.
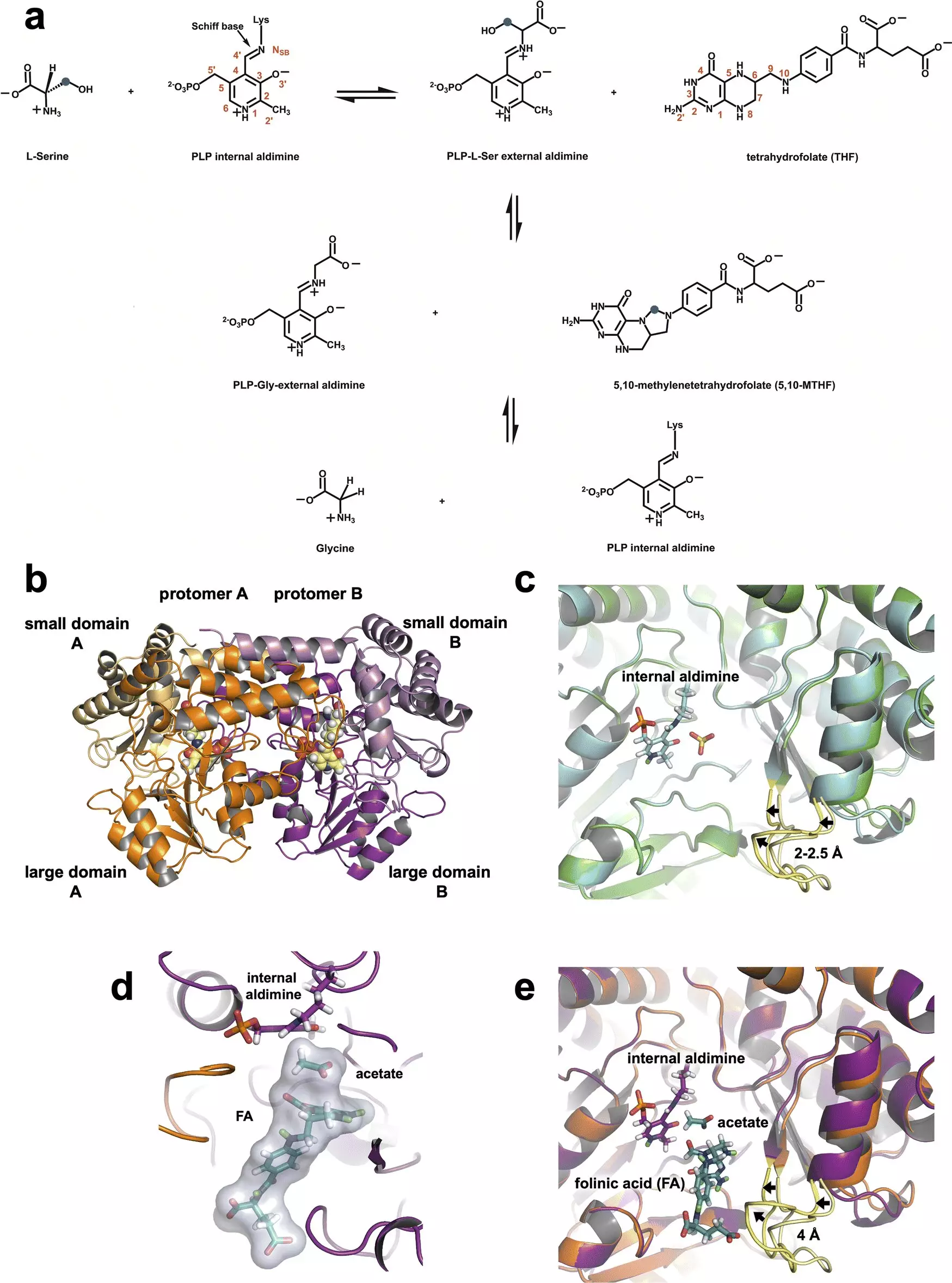
SHMT is pivotal in the one-carbon metabolism pathway, functioning primarily within cell mitochondria to convert the amino acid serine into glycine. This reaction is vital for the synthesis of nucleic acids—crucial components of DNA and RNA—as well as other biomolecules necessary for cell division. In a cancerous context, SHMT’s activity becomes dysregulated, effectively turning it into a catalyst for tumor growth by promoting rapid cell proliferation. This raises the stakes for researchers to develop inhibitors that can disrupt SHMT’s function, potentially stalling the aggressive replication of cancer cells.
The research leveraged two neutron sources—the Spallation Neutron Source and the High Flux Isotope Reactor—to shine light on the enzyme’s structure and function at an atomic level. This use of neutron diffraction is groundbreaking, as neutrons can provide insights into light elements such as hydrogen, which plays a central role in many biochemical reactions. This capability is particularly vital since enzymes are predominantly composed of hydrogen atoms, allowing researchers to understand their catalytic mechanisms better.
Victoria Drago, the lead author of the study, commented on the rapid insights gained from neutron studies, emphasizing their potential in future drug design strategies. The research not only confirmed existing hypotheses about SHMT’s function, but also revealed the critical role of a specific amino acid—glutamate—in regulating the enzyme’s catalytic activity. The ability of glutamate to both accept and release protons allows it to participate dynamically in chemical reactions, showcasing the complex nature of enzymatic processes.
For decades, researchers have grappled with the intricacies of SHMT’s catalytic mechanism and its active site dynamics. Previous findings had hinted at the importance of various amino acids, but the exact contributions remained contentious. The recent study has put significant debate to rest by demonstrating that the proton-carrying ability of glutamate is central to SHMT’s functionality. This clarity could not only reshape our understanding of SHMT but could also set the stage for the development of novel therapeutic agents targeting this enzyme.
The implications of this research extend far beyond theoretical understanding. By identifying hydrogen atom locations and their influence on the enzyme’s active sites, the researchers have provided invaluable information that could facilitate the design of small-molecule inhibitors. These inhibitors could potentially replace tetrahydrofolate in SHMT’s interactions, effectively halting the metabolic processes required for cancer cell growth. This represents a significant advantage over conventional chemotherapeutic strategies that usually target later stages of metabolism.
Cancer treatment has historically faced challenges due to the complexity and adaptability of cancer cells, which can circumvent therapeutic strategies by altering metabolic pathways. The findings related to SHMT offer a promising entry point to preemptively disrupt these processes before tumors can actively grow. This early intervention strategy could potentially lead to more effective treatments with fewer side effects, as the inhibitors would be targeting a crucial enzyme much earlier in the metabolic cascade.
Continuing advancements in neutron scattering technologies, such as stronger proton beams, have made it possible to derive quicker and more detailed data from smaller samples. This trend bodes well for future research, allowing scientists to expedite their understanding of complex biochemical systems that contribute to diseases like cancer. As William Nelson from the Sidney Kimmel Comprehensive Cancer Center pointed out, we are inching closer to a realm where artificial intelligence can assist in customizing drug therapies based on individual genetic makeup.
While we may not yet be at a point where rapid, fully personalized cancer treatments exist, the findings from this research represent a step towards that goal. By utilizing cutting-edge methodologies to study the intricate workings of enzymes like SHMT, scientists are paving the way for more targeted and effective therapeutic approaches against cancer, heralding a new era in the fight against this pervasive disease.