As global temperatures soar and climate patterns shift, the implications of high atmospheric carbon dioxide (CO2) concentrations are increasingly alarming. Beyond evident climate issues, triaging the environmental health of our planet reveals a more subtle yet critical aspect: the direct consequences of CO2 on cellular function. Recent studies underscore that CO2 interacts with biological compounds in ways that may jeopardize human health—a less discussed yet crucial component in the narrative surrounding climate change.
Ohara Augusto, a leading researcher from the University of São Paulo’s Chemistry Institute (IQ-USP) in Brazil, examines this relationship in detail. His work suggests that increased CO2 levels could contribute to various physiological dysfunctions due to changes in cellular chemistry. Specifically, the gas reacts with hydrogen peroxide (H2O2), leading to the formation of peroxymonocarbonate, a powerful oxidant. The development of techniques to detect this compound in human cells has garnered attention, particularly in the context of its potential role in cellular signaling and dysfunction.
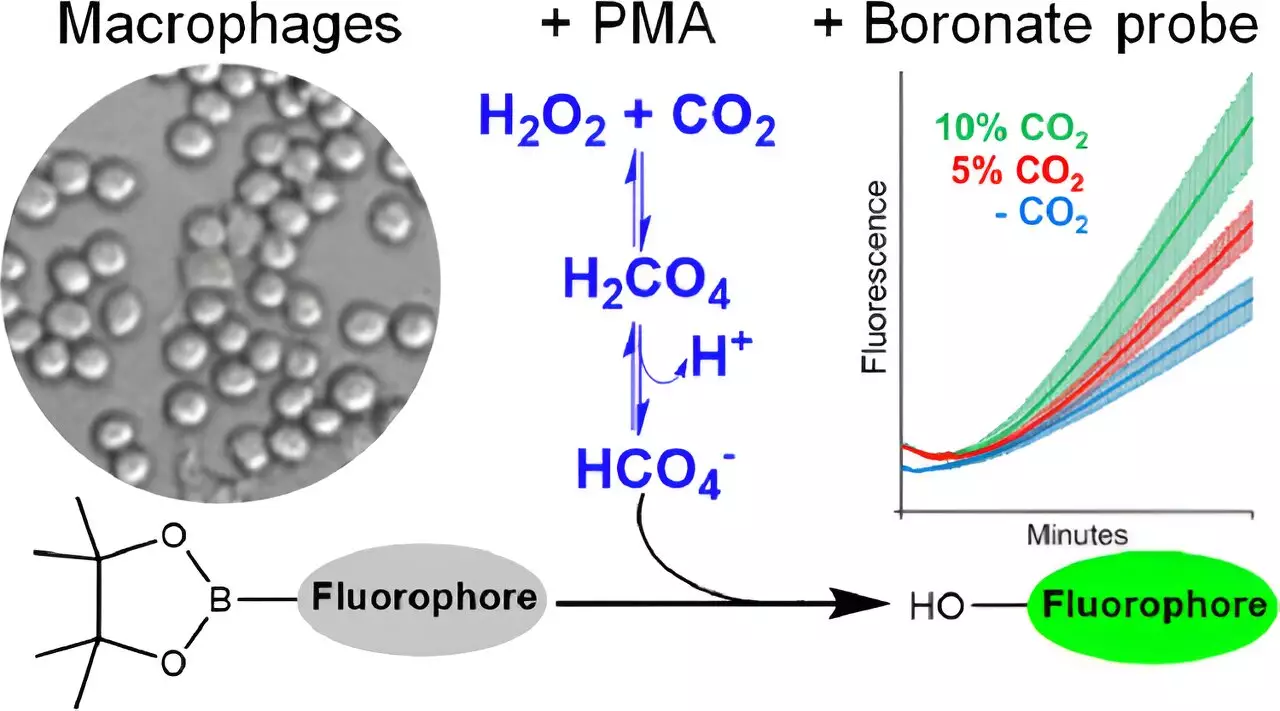
The newly developed research published in *Chemical Research in Toxicology* details a groundbreaking method for visualizing the production of peroxymonocarbonate within cells. By employing fluorescent molecular probes, Augusto and his team have set the stage for unprecedented exploration into the dynamics of oxidative stress. This is the first time peroxymonocarbonate has been identified within a cellular environment, a significant breakthrough that allows for deeper investigation into CO2’s ramifications on human biology.
The detection process involved meticulously controlled experiments where boronate probes were employed to measure changes in fluorescence indicative of peroxymonocarbonate production. This sophisticated approach hinges upon the interactions of various oxidants, including H2O2, peroxynitrite, and hypochlorous acid. The results bolstered the notion that CO2 can indeed catalyze the formation of peroxymonocarbonate under typical physiological conditions.
Delving deeper, the study pioneers investigations into biological systems through the lens of macrophages—immune cells proficient in generating reactive oxygen species. When subjected to conditions designed to stimulate their activity, these cells produced notable quantities of hydrogen peroxide, leading to the subsequent emergence of peroxymonocarbonate in the presence of CO2. The study successfully ruled out the generation of other oxidants like peroxynitrite, attributing the production of this potent oxidant specifically to CO2’s influence.
This clear delineation of oxidant presence allows researchers to revisit what was once considered impossible: the presence of peroxymonocarbonate in living cells, previously dismissed due to the low concentrations of its precursors.
The implications of this research stretch far beyond laboratory settings. As cellular environments experience slight oxidative stress, they often engage in adaptive responses, such as the upregulation of antioxidant enzymes. However, the findings suggest a nuanced dance between beneficial and detrimental effects. While mild oxidant formation can trigger protective mechanisms, excessive oxidation can culminate in irreversible cellular damage.
Interestingly, peroxymonocarbonate appears to play a critical role in this redox signaling, oxidizing thiol proteins more rapidly than its hydrogen peroxide counterpart. This revelation reinforces the need for more extensive studies to determine the thresholds at which oxidative signaling transforms from protective to harmful.
While carbon dioxide has long been recognized primarily as a greenhouse gas, its multifaceted roles in biological systems demand attention. The modulation of hydrogen peroxide and its involvement in various biological processes—such as gene expression and post-translational modifications—indicate that heightened CO2 levels may perturb normal cellular functions in critical and possibly harmful ways.
Although studies are in their nascent stages, there is growing evidence that peroxymonocarbonate could serve as one of the intermediaries that explains the deleterious effects of increased CO2 concentrations within the human body. More research is urgently needed to fully elucidate these mechanisms, exploring not only redox reactions but also potential non-redox pathways through which CO2 can inflict damage.
As we continue to grapple with the realities of climate change, findings like those of Augusto’s team present an undeniable call to action. Increasing CO2 levels constitute more than just an environmental threat; they infiltrate our biology and potentially imperil human health. There exists an imperative to deepen our understanding of how these atmospheric changes translate into cellular dynamics, framing a holistic view of climate actions that converge on both environmental sustainability and public health.
Addressing the risks associated with prolonged exposure to elevated CO2 concentrations may ensure that as we advocate for cleaner air, we are also safeguarding the intricate biological systems that sustain human life. In doing so, we not only contribute to a healthier planet but also fortify our own health against the cascading effects of a changing climate.