Cholesterol, a vital molecule in cell biology, plays an indispensable role in the structure and function of biomembranes. These membranes are complex assemblies of proteins and lipids, where cholesterol is not merely a filler but a critical component that influences the fluidity and organization of these membranes. Understanding the nuances of how cholesterol interacts within cellular membranes has eluded scientists, primarily due to its intricate structure and behavior under different conditions. A recent study conducted by researchers at Rice University, spearheaded by Jason Hafner, offers significant insights into this long-standing scientific enigma, potentially paving the way for new therapeutic strategies in the treatment of diseases such as cancer, where membrane integrity is paramount.
The team’s groundbreaking research, which was detailed in the Journal of Physical Chemistry, leveraged Raman spectroscopy to investigate the unique characteristics of cholesterol embedded in biological membranes. Unlike traditional methods, Raman spectroscopy employs laser light to probe molecular vibrations, allowing researchers to gather detailed information about the molecular environment. This innovative approach enabled Hafner and his team to examine various cholesterol conformations directly within their native settings, a feat that had not been accomplished prior to this study. By comparing experimental spectra with those predicted by density functional theory, which incorporates advanced quantum mechanical computations, the researchers were able to unravel the complexities of cholesterol’s interactions in membranes.
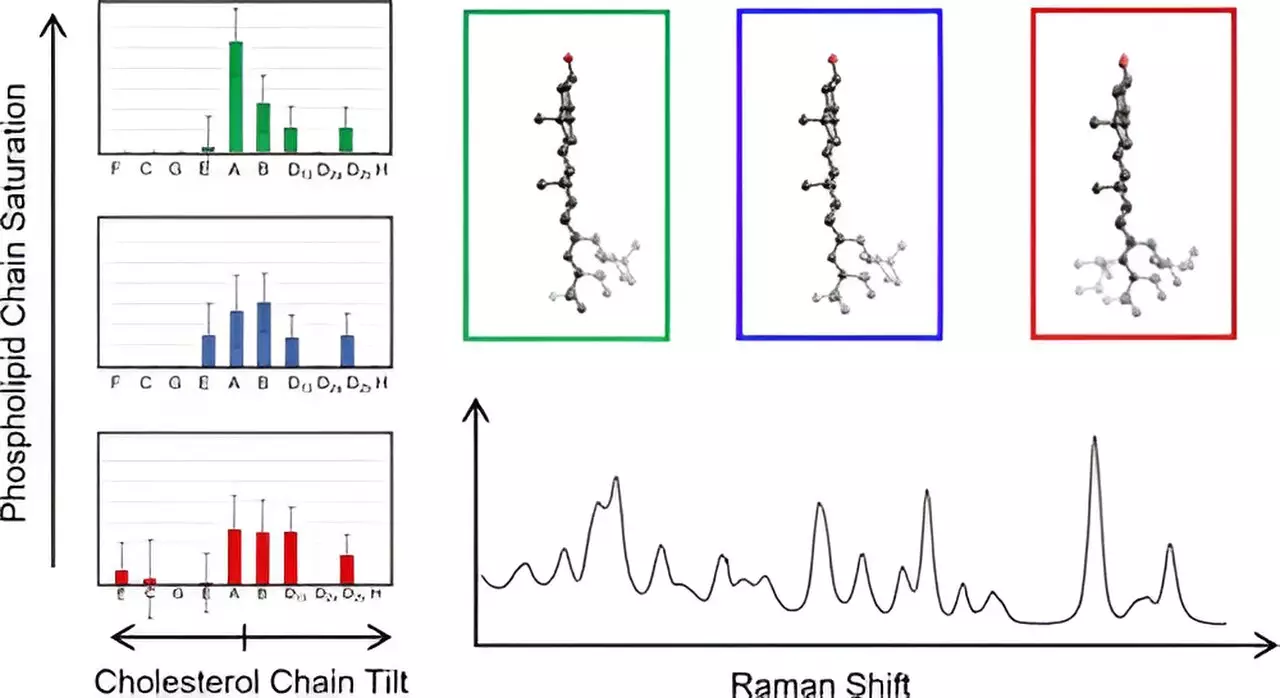
Hafner’s research uncovered that cholesterol molecules could be categorized based on the deviations of their carbon chains from the planar ring structure that is a hallmark of cholesterol. This classification not only identifies previously unrecognized structural variations but also fundamentally alters the understanding of how cholesterol behaves in membrane contexts. Impressively, the study revealed that cholesterol molecules with similar structural attributes exhibited identical low-frequency spectra, suggesting a level of uniformity that simplifies the analysis of these molecules in biological membranes. Such findings highlight the potential for advancements in biomolecular research and open avenues for investigating the consequences of disrupted cholesterol distribution in disease states.
Given the critical implications of Hafner’s findings on the study of cellular membranes, future research may focus on the potential links between cholesterol distribution and various diseases. As cancers and other pathological conditions often exploit aberrations in membrane structure, a deeper understanding of cholesterol’s role could lead to innovative biomarkers or therapeutic avenues. Furthermore, the methodologies established through this research lay a robust foundation for subsequent studies aimed at dissecting the molecular dynamics of other essential biomolecules in the context of cellular function. This study not only confirms the significance of cholesterol in cellular environments but also serves as a model for employing advanced analytical techniques in membrane biology.
The pioneering work led by Hafner and his team has illuminated the intricate dance of cholesterol within cell membranes, highlighting its fundamental role and potential implications for human health. As researchers continue to explore this fascinating area of study, we may soon unlock new strategies for combating diseases associated with membrane dysfunction.