Organic chemistry continuously strives for innovation, particularly in the arena of catalysis where efficiency and sustainability are key. Recent research spearheaded by a team at Chiba University, Japan, underlines a remarkable advancement in the use of samarium (Sm), a rare earth metal, in chemical transformations. This breakthrough suggests a paradigm shift in the application of Sm compounds, promoting not only their efficiency but also reducing the resources required for their use in real-world scenarios.
At the core of this research is samarium, particularly its ability to generate single-electron transfer reductions via its divalent compounds. Among these, samarium iodide (SmI2) stands out due to its moderate stability and effectiveness in carrying out reactions at room temperature, making it a prime candidate for generating pharmaceuticals and other biologically relevant materials. However, the engagement of SmI2 comes at a cost; conventional reactions demand stoichiometric amounts of the compound, often combined with toxic chemicals that heighten both resource intensity and expense.
This scenario underscores the necessity for more sustainable solutions. Research efforts have explored various strategies aimed at minimizing the use of Sm reagents, yet many of these methodologies are hampered by the need for harsh conditions and reactive reducing agents. As a result, they still require 10–20% of raw materials to effectively yield results. The pressing expense of samarium, coupled with this extensive resource requirement, amplifies the demand for an efficient catalytic system that could operate under milder conditions while utilizing significantly lower quantities.
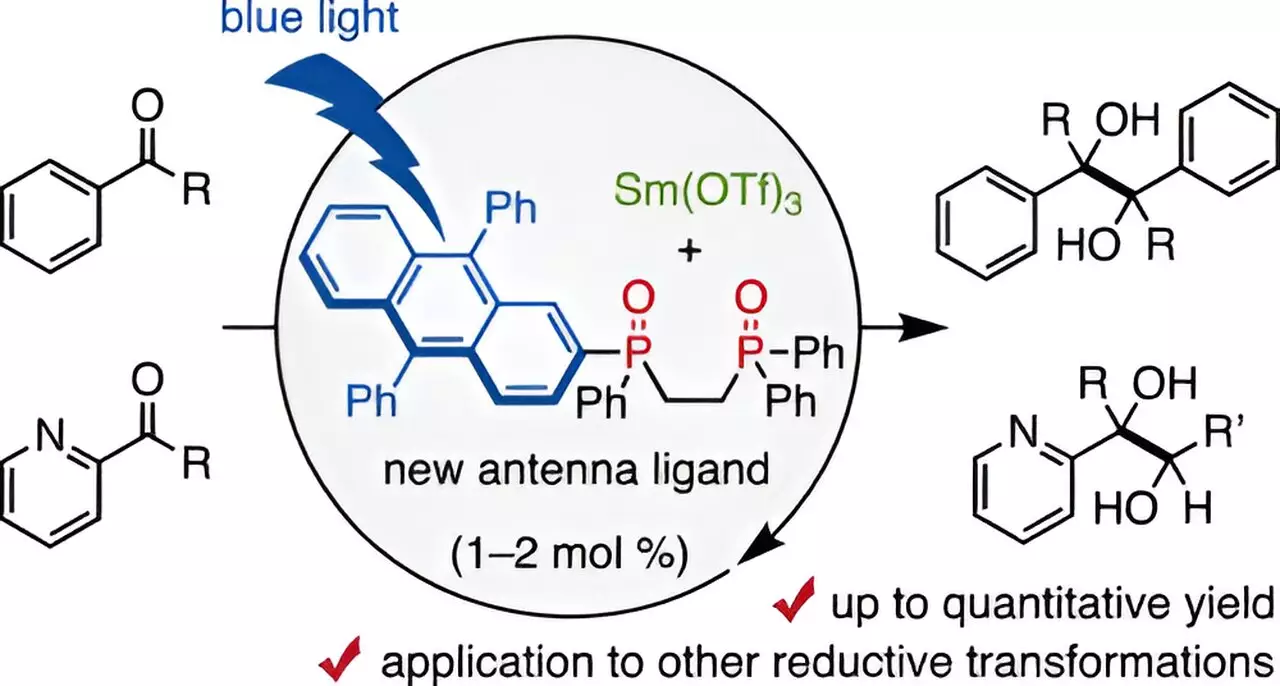
The recent findings from Chiba University mark a significant breakthrough in achieving this goal. The research team, led by Assistant Professor Takahito Kuribara, unveiled a unique catalytic method that utilizes a novel 9,10-diphenyl anthracene (DPA)-substituted bidentate phosphine oxide ligand, which they refer to as a “visible-light antenna.” This innovative ligand facilitates the coordination of trivalent samarium, allowing for catalytic reactions to be powered by visible light.
This concept of an “antenna ligand” is pivotal; it builds on previous work that demonstrated how secondary phosphine oxide ligands could engage in reduction-oxidation reactions under visible light. The ingenuity lies in the design of the new ligand, which not only minimizes the required amount of Sm but also channels energy from blue light effectively into the reaction mechanism.
Through rigorous experimentation, the Chiba University team found that the combination of Sm and the DPA-1 ligand under blue-light irradiation could produce astonishing yields—up to 98% in pinacol coupling reactions, which are essential in pharmaceutical synthesis. This is a radical departure from traditional techniques that necessitate much higher quantities of Sm, with the new approach only requiring 1–2 mol% of the catalyst.
The simplicity of the method is underscored by its adaptability to milder organic reducing agents, unlike the previously required stronger agents that could impede the reaction process. Interestingly, the study revealed that introducing small amounts of water enhanced reaction outcomes, while excess water produced deleterious effects. In contrast, analogous ligands like DPA-2 showed poor results, emphasizing the specificity of DPA-1 in facilitating these reductive transformations.
To unravel the workings of DPA-1, the researchers meticulously examined the emission characteristics of the Sm catalyst paired with the ligand. They demonstrated that DPA-1 operates as a multifunctional entity—simultaneously coordinating with Sm, selectively absorbing blue light, and ensuring efficient electron transfer to the metal catalyst. This multifunctionality positions the visible-light antenna as a cornerstone in the future of organic reactions, especially for those involved in critical drug development processes.
The significance of this research extends beyond mere theoretical interest; it paves the way for the broader utilization of Sm-based catalysts in organic transformations. The stability offered by trivalent Sm offers practical handling benefits compared to its divalent counterpart, enhancing the feasibility of integration into various industrial applications. Moreover, the seamless incorporation of visible light as a benign energy source outlines a clear pathway toward sustainable chemical synthesis.
The innovative research led by Assistant Professor Takahito Kuribara and his team represents a pivotal advancement in organic chemistry, promising not only higher efficiency but also a reduction in the ecological footprint of chemical synthesis involving samarium. The ability to achieve high yields with minimal Sm under mild conditions enhances the potential for developing greener chemistry methods, making this breakthrough a crucial step toward sustainable practices in organic synthesis.