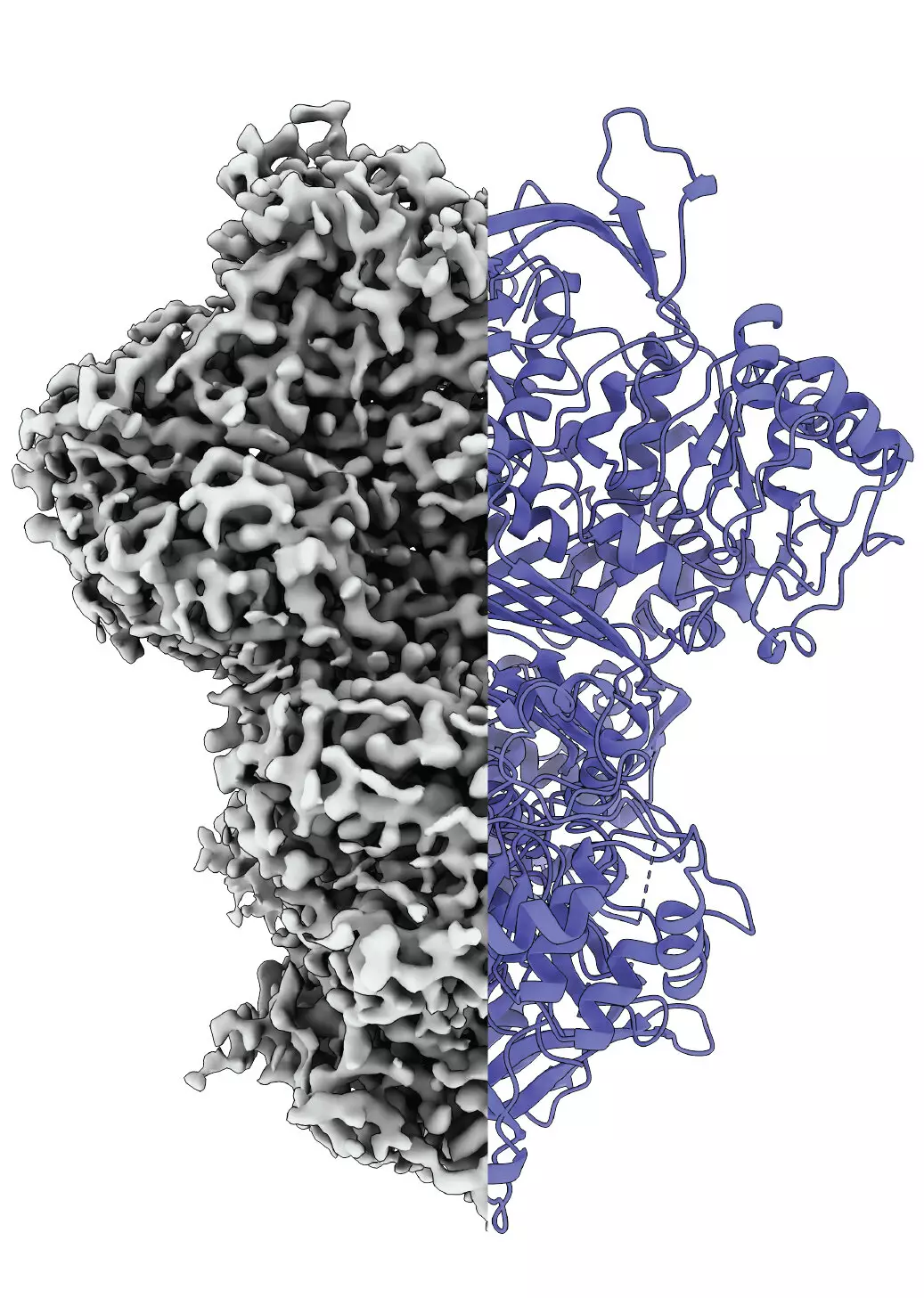
Proteins are the backbone of biological processes, acting as enzymes, structural components, and signaling molecules within living organisms. The structure of a protein directly influences its functionality; this fundamental principle is at the foundation of protein research. Researchers have long observed that the misfolding or structural alteration of proteins can lead to dysfunction, and subsequently, diseases. Therefore, the study of how proteins undergo conformational changes is crucial to understanding their roles in health and disease. A recent study has shed new light on one such protein, myo-inositol-1-phosphate synthase (MIPS), revealing its transformational nature as it transitions from a disordered to an ordered state.
A collaborative team of researchers from Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center has successfully visualized the dynamic structural changes of MIPS during its activation phase. Utilizing cutting-edge cryo-electron microscopy, they have identified that MIPS does not remain static as it fulfills its role in the production of inositol, commonly referred to as vitamin B8. This insight marks a significant stride in the field, particularly because proteins like MIPS often lack fixed structures. The research team has explored this protein in a model organism, the fungus Thermochaetoides thermophila, to analyze its functional dynamics under conditions that closely resemble those found in nature.
MIPS is a critical enzyme within a broader metabolic pathway responsible for producing inositol. What makes inositol unique is that although it is often termed a vitamin, the human body synthesizes it internally, complicating its classification. Inositol plays a plethora of roles in biological systems, including its function in cellular signaling processes, lipid metabolism, and growth regulation. Given that MIPS is integral to this operational pathway, any disruption in its function could have far-reaching consequences for cellular health and metabolism.
One of the pivotal discoveries of the research team is the identification of at least three distinct states of MIPS: a disordered state, an ordered state, and an unknown intermediate state. While the disordered phase has been known to be essential for the protein’s flexibility and interaction with substrates, the newly identified intermediate state remains enigmatic. Researchers speculate that it may facilitate essential functions, such as improved hydration and enhanced enzymatic reactions. The complexity of these conformational states highlights not only the protein’s adaptability but also the sophisticated mechanisms underlying protein behavior.
The findings on MIPS set the stage for further exploration into the behavior of other isomerases, a class of enzymes to which MIPS belongs. By analyzing structural data from over 340 isomerases, the researchers found that similar dynamic behaviors might be present in these proteins. This opens the door to a better understanding of metabolic pathways and their related enzymes, which could provide novel therapeutic avenues. By identifying specific mechanisms at play in proteins like MIPS, researchers may pave the way for innovative treatments targeting metabolic disorders or diseases linked to protein misfolding.
This research marks a significant step forward in how we understand the dynamic nature of proteins and their roles within metabolic processes. The ability to visualize MIPS as it transitions through various structural states not only enhances our foundational knowledge of protein biochemistry but also emphasizes the importance of studying proteins in environments that mimic their physiological conditions. As the scientific community continues to unravel these complex mechanisms, we can anticipate advancements in therapeutic strategies that address metabolic issues, thereby enhancing human health. The investigation of MIPS and its structural dynamics is a reminder of the intricacies of life at the molecular level, where even subtle changes can hold substantial implications for both health and disease.