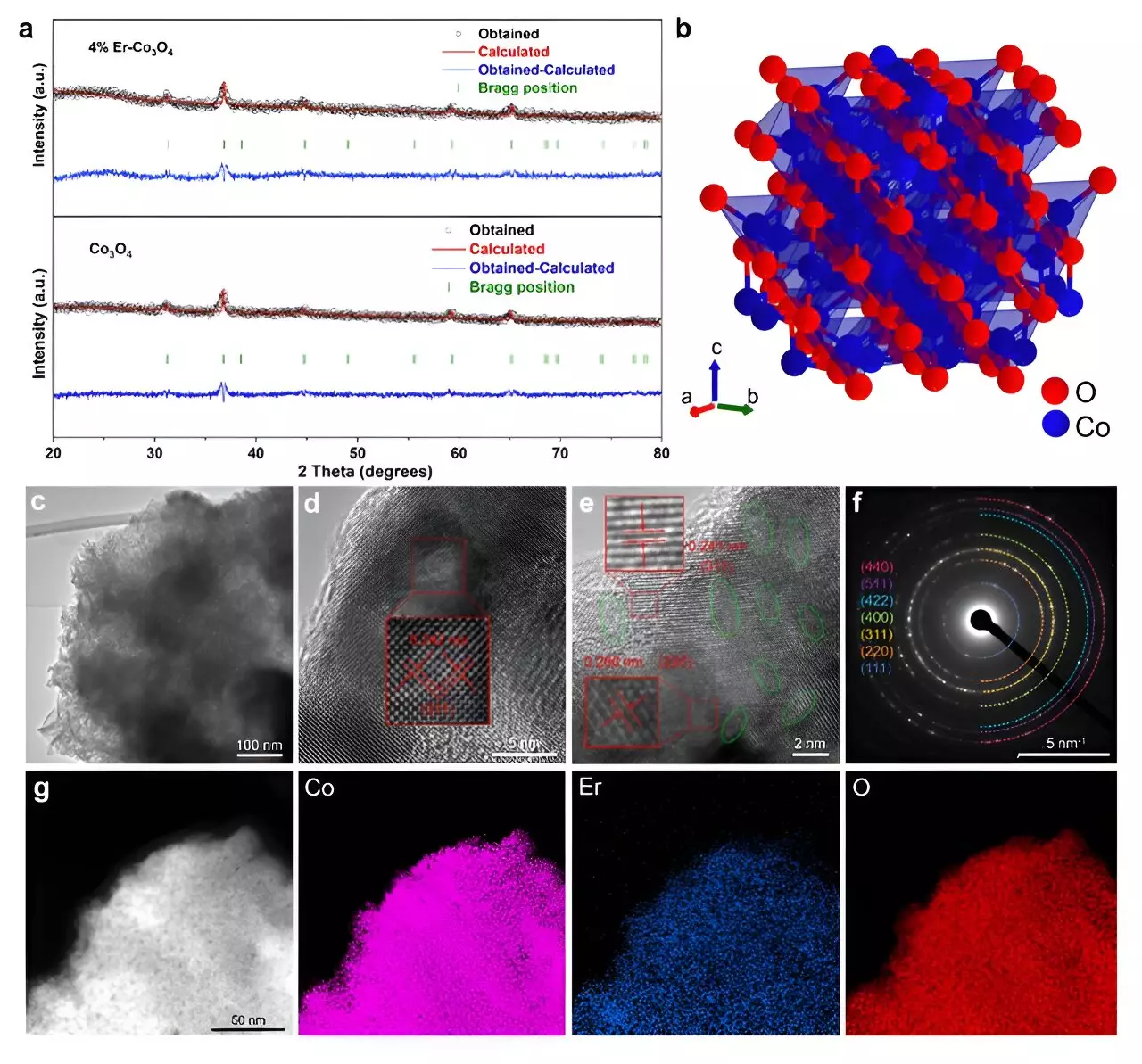
Recent advancements in electrocatalysis have sparked considerable interest in the scientific community, particularly the oxygen evolution reaction (OER), which plays a pivotal role in sustainable energy technologies such as water splitting and fuel cells. Researchers are continually searching for efficient and stable catalysts that can withstand harsh acidic environments, an essential criterion for improving the effectiveness of these reactions. A groundbreaking study involving the doping of cobalt oxide (Co3O4) with erbium (Er), a rare earth element, has emerged as a promising strategy to enhance both performance and stability, presenting a viable alternative to noble metal catalysts.
The research conducted by a team from Tohoku University’s Advanced Institute for Materials Research represents a meaningful leap in catalyst design. In a detailed study published in the reputable journal ACS Catalysis and recognized as an Editors’ Choice, the team highlights the remarkable enhancements achieved by incorporating a small percentage of erbium into the traditional Co3O4 structure. The new erbium-doped catalyst exhibited an impressive overpotential of merely 321 mV at a current density of 10 mA cm², a significant milestone that positions it competitively against conventional iridium-based options, known for their high costs.
Traditionally, cobalt oxide catalysts have been limited in their efficiency due to inherent structural challenges, particularly related to their spinel crystal forms. However, the introduction of Er fundamentally alters the electronic properties and structural attributes of the Co3O4 matrix, thereby addressing these inherent limitations. The researchers defined this improvement as critical to achieving optimal catalytic activity in demanding environments.
Mechanistic Insights: Why Does Doping Work?
To thoroughly understand the underpinnings of this enhanced performance, the research team utilized both microkinetic modeling and density functional theory (DFT) calculations. This analytical approach unveiled that the addition of erbium increases the ratio of Co3+ to Co2+ ions within the catalyst, a condition that is essential for optimizing reaction kinetics associated with the OER. This process results in the generation of oxygen vacancies—sites crucial for the electrocatalytic reaction to proceed swiftly.
Co-author Tianyi Wang articulated this mechanism through an insightful analogy likening the catalyst to a multi-lane highway, where the introduced erbium serves to create additional pathways for the migration of reaction intermediates, thus enhancing catalytic throughput.
Structural Advantages of Erbium Doping
A further investigation using in situ Raman spectroscopy revealed that the oxygen vacancies formed due to erbium doping were predominantly localized within the octahedral sites of the Co3O4 structure. These vacancies not only facilitate the formation of key intermediates in the OER process but also bolster the overall catalytic activity by providing additional mobile Co3+ sites necessary for binding these critical intermediates.
Wang emphasized the significance of the interplay between electronic enhancements and structural modifications, stating that this balance is pivotal for high catalytic performance. The research highlights the necessity of considering these factors in the pursuit of advanced electrocatalyst design.
The findings from this research have profound implications for the future of electrocatalysis. The integration of low-cost, non-precious metal components into catalyst design stands to revolutionize the field by making high-performance catalysis broadly accessible and economically viable. The promise of using inexpensive materials without compromising efficiency tackles both environmental sustainability and economic challenges associated with precious metal dependence.
As the interest in green technologies accelerates globally, this research sets a precedent for exploring further avenues in the development of durable and efficient electrocatalysts. The team aims to broaden their investigation into other non-precious metals, laying the foundation for sustainable solutions that cater to the increasing energy demands of the modern world.
The incorporation of erbium into cobalt oxide catalysts marks a substantial advancement in electrocatalytic research. By enhancing both stability and efficiency, this innovative approach not only facilitates the OER but also contributes to the broader agenda of achieving sustainable energy solutions. The continued exploration of cost-effective catalysts paves the way for a greener future, prioritizing both performance and environmental responsibility. As researchers embark on this promising journey, the implications of these findings resonate across the field, inspiring the next generation of energy solutions.