Imagine if you could open a flat-pack box of furniture from IKEA and see all the pieces come together to form a beautiful chair without having to screw a single bolt. This whimsical notion is not as far-fetched as it sounds, especially when we consider the principles governing self-assembly in chemistry and biology. The spontaneous organization of small building blocks into complex structures is a phenomenon observed across various biological systems, including proteins and cell membranes. Researchers are increasingly tapping into these principles to enhance materials science through supramolecular chemistry, a discipline focused on creating larger molecular structures from simpler components.
Supramolecular chemistry allows scientists to manipulate the interactions between molecules, leading to the creation of “smart materials” that can adapt to their environment. By adjusting the forces acting between different polymers, researchers can effectively create materials that respond dynamically to stimuli such as heat or the introduction of new chemicals. Despite advancements, however, many elements of supramolecular assembly still elude comprehensive understanding. A recent study from Osaka University provides enlightening insights into this intricate world by exploring how additives can influence the self-assembly of spherical microparticles derived from poly(sodium acrylate).
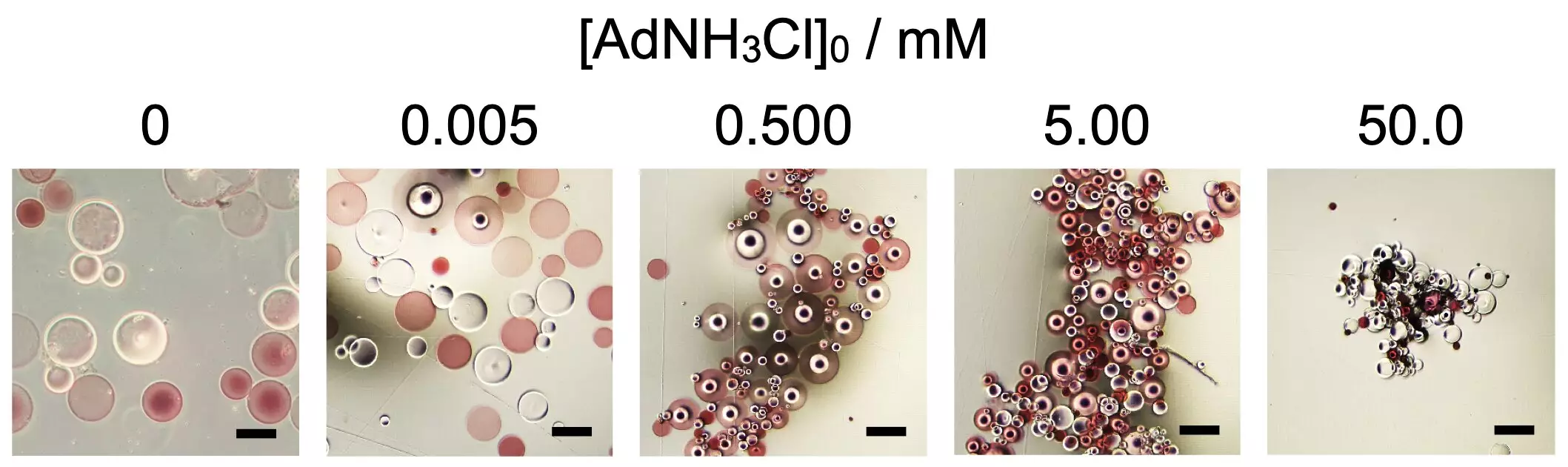
The Osaka University research employed a specific class of functionalized polymers — those altered with β-cyclodextrin and adamantane residues — to investigate their self-assembly capabilities. Notably, it was found that only when a critical concentration of the additive 1-adamantanamine hydrochloride (AdNH3Cl) was introduced did these microparticles begin to merge. This phenomenon parallels the behavior of biological molecules, where interactions among amino acids dictate the folded configurations of proteins. The study’s lead investigator, Akihito Hashidzume, articulated that the complex interplay of attraction and repulsion among molecules is fundamentally the same across living organisms.
The implications of this research extend beyond mere academic curiosity. The ability to modulate the macroscopic shapes and functions of assemblies based on varying concentrations of stimuli presents exciting possibilities. For example, researchers identified that the shape of the assembled microparticles could be adjusted to be more spherical or elongated depending on the concentration of the additive, suggesting a robust mechanism for controlling material properties at the microscale. Senior author Akira Harada noted that understanding these mechanisms might shed light on the formation of diverse biological shapes, potentially revolutionizing how we approach the design of active materials.
The exploration of self-assembly within supramolecular chemistry offers profound insights into how we understand and manipulate materials on both microscopic and macroscopic levels. As scientists continue to unravel the complexities of these interactions, we can anticipate significant advancements that not only enhance our basic understanding of molecular biology but also pave the way for innovative materials that respond intelligently to their environments. The delicate dance of molecules, as nature demonstrates, holds the key to a plethora of applications in material science and biotechnology, with each discovery leading us closer to practical implementations that could change our interaction with the material world.