Heavy metal contamination in water sources poses a significant threat to both human health and the ecosystem. Metals such as cadmium and lead, even at low concentrations, can lead to severe health issues, including neurological damage and developmental disorders. Not only do these pollutants endanger drinking water quality, but they also disrupt aquatic life, causing long-lasting environmental damage. Traditional methods of purification, such as filtration and membrane technologies, often require vast amounts of energy and frequently lead to clogged systems, necessitating costly replacements and maintenance.
Additionally, heavy metals are persistent in the environment, making their removal a constant hurdle for environmental scientists and engineers alike. Recent innovations in water purification technologies have pointed toward the utilization of plant-derived materials, specifically polysaccharides. These naturally occurring polymers have shown promise in creating sustainable solutions for removing heavy metals from contaminated water bodies, but they often arrive with challenges related to their solubility and stability.
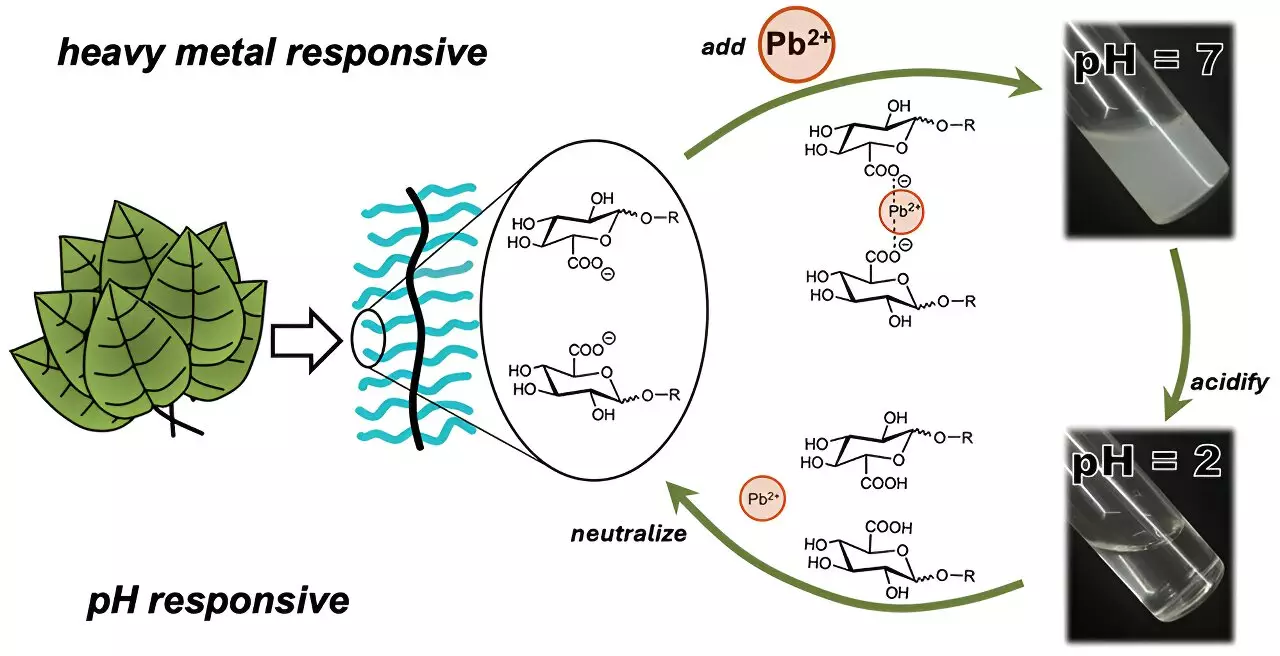
A groundbreaking advancement made by researchers at the University of Texas at Austin offers a promising new avenue for addressing heavy metal contamination. This team, under the guidance of Cassandra Callmann, developed a novel sugar-derived polymer that effectively binds heavy metal ions into insoluble clusters. This polymer does not merely function as a passive filter; it engages in active binding with specific ions, thereby enhancing its efficiency in water purification processes.
At the core of the innovation is the construction of a polymer backbone that is insoluble in water, adorned with dangling carbohydrate moieties that exhibit varying solubility characteristics. The ingenious design allows different carbohydrate “charms” to respond to specific heavy metal ions like cadmium and lead, creating an interactive filtration system capable of isolating undesirable contaminants.
Initial laboratory tests have illustrated the polymer’s powerful capabilities. One of the significant findings was that the carbohydrate “charm” containing a carboxylic acid group demonstrated remarkably high binding efficiency to ionic cadmium. Within a mere three minutes of exposure to contaminated water, this polymer formed visible clumps which could be promptly filtered out, showcasing its practicality in real-world applications.
What makes this development particularly valuable is the polymer’s ability to readjust and release the captured metal ions through variations in water acidity. This recyclability not only boosts the efficacy of the filtering process but potentially lowers costs associated with frequent material replacement. Such a system could transform water treatment facilities by allowing them to utilize the same polymer multiple times while maintaining high performance levels.
Taking their research a step further, the team conducted tests using contaminated samples from the Colorado River. The results were promising; during a 24-hour testing window, the polymer managed to capture up to 20% of the added cadmium and an impressive 45% of the lead. Even more encouraging was its minimal interaction with naturally abundant metal ions, such as calcium, sodium, and magnesium, which typically could complicate filtration processes.
These outcomes indicate a significant leap forward in creating selective, efficient materials for water purification. The ability to distinguish between harmful heavy metals and beneficial ions is critical for ensuring that water treatment is both effective and environmentally responsible.
The development of this innovative sugar-like polymer represents a significant advancement in the fight against water pollution. Its unique binding mechanism, combined with its adjustable solubility, suggests that it could revolutionize traditional water treatment processes. Moving forward, researchers aim to further refine the polymer’s design, optimizing its efficiency and scalability for broader applications.
With the ever-increasing threat of heavy metal contamination worldwide, this technology offers a beacon of hope. It exemplifies how plant-derived materials can pave the way for sustainable solutions, merging environmental stewardship with cutting-edge scientific advancement. As we continue to confront the challenges of water quality and safety, innovations such as these provide a clear pathway toward a healthier future for our communities and ecosystems.