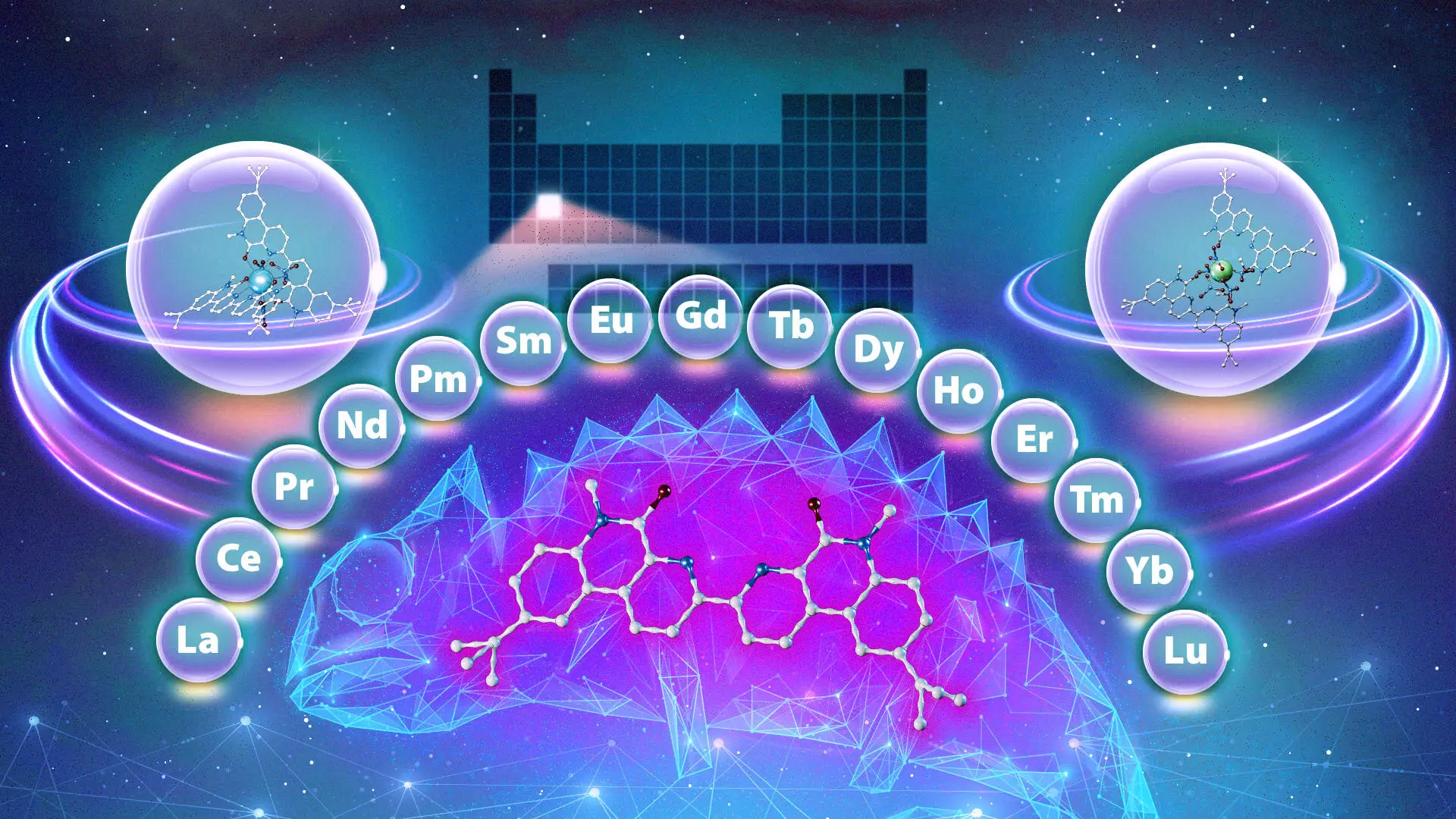
The increasing global demand for clean energy technology, medical devices, and advanced electronics has magnified the importance of rare-earth metals, particularly lanthanides. Recent research from the Department of Energy’s Oak Ridge National Laboratory (ORNL) in collaboration with Vanderbilt University has introduced a novel chemical compound termed a “chameleon,” which offers promising enhancements to the extraction of these essential metals. This groundbreaking study highlights the ongoing commitment of the scientific community to overcome challenges in accessing lanthanides, thereby facilitating their broader application in various industries.
Despite their name, rare-earth metals are not inherently rare in terms of their natural abundance. In fact, lanthanides are often found in mineral deposits, comparable in prevalence to more familiar metals such as copper and lead. The term “rare” relates more to the difficulty involved in their extraction and purification rather than their actual availability in nature. Collectively, there are 17 elements designated as rare-earth metals, with lanthanides playing a pivotal role in modern technology. Their unique electronic properties make them essential for applications ranging from magnetic materials to catalysts in chemical reactions.
One of the paramount challenges in utilizing lanthanides stems from their similar physical and chemical characteristics. The lanthanide ions’ sizes and properties vary only slightly, complicating the extraction and purification process. Efficiently isolating these metals requires sophisticated techniques that can accurately differentiate among these closely related elements.
Current industrial methodologies for separating lanthanides depend heavily on the use of ligands—molecules that can selectively bind to specific metals. These ligands are used in conjunction with organic solvents, initiating a separation process based on varying solubility in water. However, the conventional extraction techniques are labor-intensive, costly, and generate significant environmental waste due to the multi-step nature of the separation process.
Typically, lanthanide separation occurs in a predetermined order, based on either their weight — from heavy to light or vice versa. This systematic approach often produces challenging waste management issues and consumes considerable time and energy. The research highlighted in this article presents an innovative remedy by introducing a ligand with adaptable properties that could streamline the entire process.
Initial investigations surrounding existing ligands yielded surprising results, revealing a compound that demonstrates variable binding characteristics contingent upon its surrounding conditions. Similar to a chameleon altering its color, this ligand adapts its binding preferences based on the acidity of the solution and the duration of its interaction with lanthanide ions. For instance, under more acidic conditions, the ligand favors the binding of heavier lanthanides.
This unique mechanism permits the same ligand to target various lanthanides effectively throughout the separation process, a significant departure from traditional systems that typically favor either lighter or heavier ions exclusively. According to co-lead researcher Santa Jansone-Popova, this novel behavior of the chameleon ligand could drastically reduce the number of steps required to separate lanthanides, ultimately leading to more efficient and environmentally sustainable extraction techniques.
Furthermore, this research suggests that the chameleon ligand could selectively extract lanthanides in any order, a remarkable capability that could revolutionize current practices.
Despite the promising findings, the journey does not end here. The discovery of the chameleon ligand opens the door to further exploration and development of ligands exhibiting similar adaptable properties. Understanding the underlying mechanisms driving this variability in behavior presents an exciting opportunity for innovation in ligand design, providing the potential for new compounds that can enhance the separation of lanthanides more effectively.
The implications of this research stretch beyond enhancing extraction efficiency. As industries strive for greener practices, a method that generates less waste and utilizes fewer resources aligns closely with global sustainability goals. Advancing the capacity to access and purify rare-earth metals effectively plays a crucial role in supporting the transition towards cleaner energy technologies and improvements in various sectors such as healthcare and electronics manufacturing.
The discovery of a chemical chameleon at ORNL represents a significant step in tackling the complex challenges surrounding rare-earth metal extraction. By developing adaptable ligands that enhance separation efficiency, the scientific community is paving the way for a more sustainable and accessible future for critical materials essential to modern technology.