In recent developments from RIKEN, chemists have shed light on the substantial potential of dinitrogen (N2)—a molecule abundant in our atmosphere—for innovative chemical synthesis. Comprising nearly 80% of Earth’s air, dinitrogen’s accessibility contrasts sharply with the challenges it poses in practical application. The traditional methods of harnessing dinitrogen for synthesizing vital compounds—ranging from pharmaceuticals to various industrial materials—have often proven to be energy-intensive and complicated. Thus, the current work represents a pivotal move toward improving the efficiency and sustainability of chemical manufacturing processes.
Utilizing dinitrogen in chemical processes is hindered by the strong triple bond present in the molecule, making its direct use exceedingly rare and complex. Standard approaches like the Haber-Bosch process initiate the conversion by first disassembling the N2 molecule into ammonia—a procedure that, while effective, requires substantial energy input. Furthermore, the immediate precursor for synthesizing alkyl amines, such as alkenes, necessitates pre-activation, a step that further complicates the reaction chain and adds to the energy expenditure. This multi-step process is not only inefficient but also contributes significantly to the overall carbon footprint of the synthesis.
The researchers at RIKEN, led by Takanori Shima from the RIKEN Center for Sustainable Resource Science, emphasize the dire need for innovative solutions that allow for the direct utilization of dinitrogen and alkenes under milder conditions. This catalytic transformation could pave the way for a new era in synthetic chemistry, making processes more straightforward, cost-effective, and environmentally friendly.
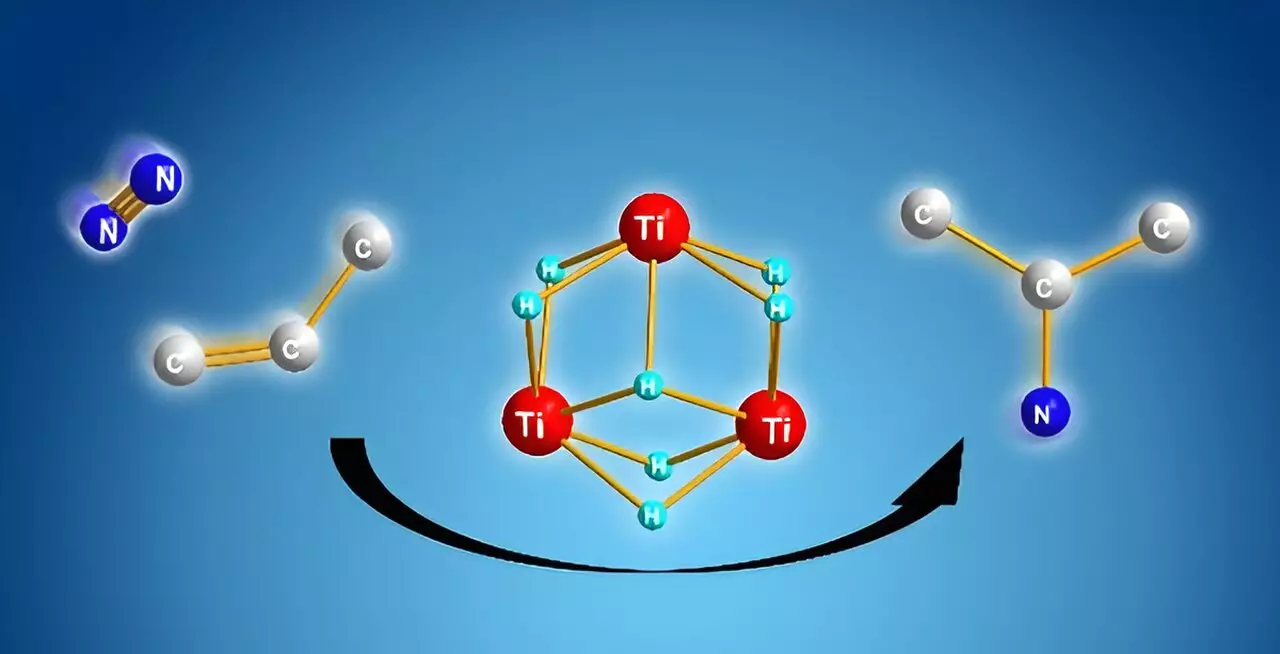
To address the limitations posed by traditional methodologies, Shima and colleagues turned towards titanium polyhydrides—complexes comprising titanium atoms linked by hydrogen. Previous research had already suggested that these compounds exhibit exceptional reactivity with inert molecules like benzene and dinitrogen. The breakthrough application stems from their ability to facilitate simultaneous reactions that lead to the synthesis of alkyl amines from these two components.
The ingenious design of titanium polyhydrides enables cooperative interaction among multiple titanium-hydride units. During the reaction with alkenes, these units activate without fully expending their reactive capability. Subsequent addition of dinitrogen triggers a series of bond cleavages and formations, resulting in the generation of a new nitrogen-carbon bond critical for alkyl amine synthesis. Remarkably, the catalytic activity remains intact after the reaction, suggesting the potential for this method to repeat and scale more efficiently.
Crucial to this advancement is an in-depth computational analysis that elucidates the mechanisms governing these reactions. It was discovered that the formation of nitrogen-carbon bonds occurs preferentially within the titanium polyhydride framework due to its favorable energy dynamics, overshadowing other possible bond formations such as nitrogen-hydrogen or carbon-hydrogen interactions. This specificity not only ensures successful product formation but also minimizes undesired side reactions, further enhancing yield.
The implications of these findings stretch far beyond mere academic interest. Shima and his research team are now working on developing a fully catalytic process derived from their groundbreaking approach, positioning dinitrogen as a cornerstone of sustainable chemical production. The potential to create alkyl amines directly from abundant feedstocks represents a significant leap towards greener chemistry practices, benefiting various sectors including pharmaceuticals, agriculture, and materials science.
By addressing the multifaceted challenges associated with dinitrogen utilization, this innovative strategy not only advances the field of synthetic chemistry but also underscores the necessity for sustainable practices in modern industrial applications. As further research unfolds, it is likely that the chemistry community will witness a significant shift in how dinitrogen is perceived and exploited—a transformation that could have lasting implications for how we conduct chemical research and manufacturing in the decades to come.