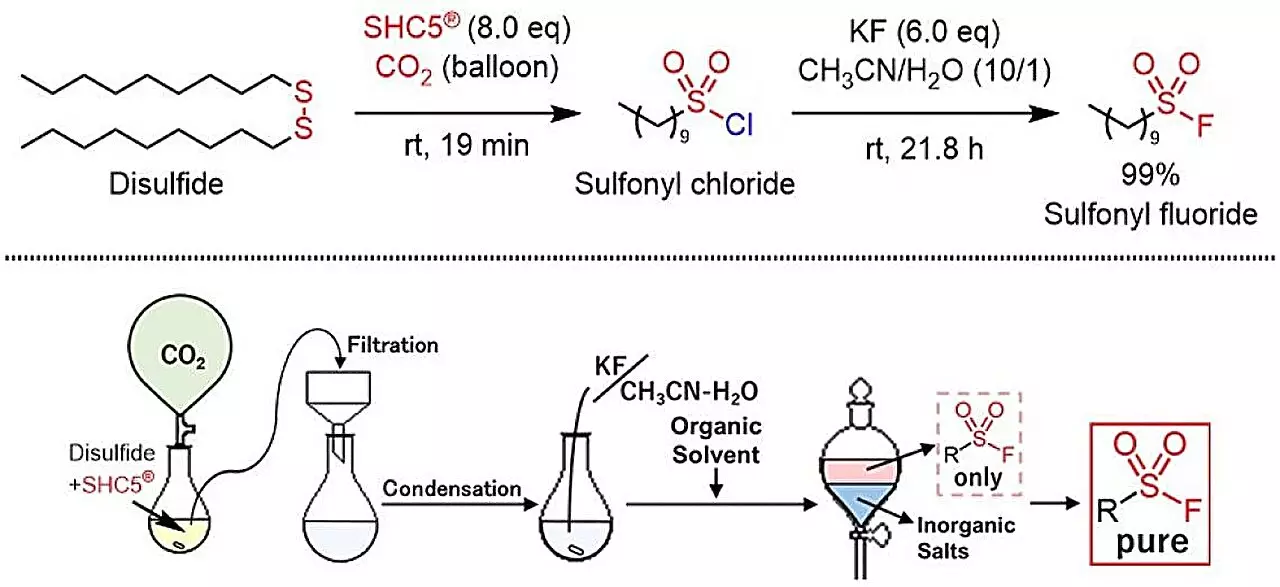
In a significant breakthrough within the field of synthetic chemistry, a novel method has been established for synthesizing sulfonyl fluorides from thiols and disulfides. Researchers have employed a combination of SHC5 and potassium fluoride (KF), a process which marks an important advancement in “click chemistry.” This innovative technique not only enhances efficiency but also prioritizes environmental sustainability, producing only sodium chloride (NaCl) and potassium chloride (KCl) as byproducts. The findings, recently published in ACS Sustainable Chemistry & Engineering, open up new avenues for chemical and industrial synthesis, potentially making this method the preferred option for researchers and companies alike.
Click chemistry has gained prominence over the years due to its remarkable capabilities for molecular assembly. It is characterized by its high selectivity, rapid reaction times, and substantial yield, which has made it indispensable in various fields, ranging from materials science to pharmaceutical development. However, the synthesis of sulfonyl fluorides, crucial for sulfur-fluorine exchange (SuFEx) reactions, has traditionally relied on toxic substances like sulfur dioxide fluoride (SO2F2) or potassium hydrogen fluoride (KHF2). These compounds are not only hazardous but also pose significant handling challenges, leading the scientific community to seek safer alternatives.
The innovative research presented in this study showcases a groundbreaking approach to synthesize sulfonyl fluorides effectively and safely. In this process, SHC5 is combined with potassium fluoride to react with readily available thiols or disulfides. The result is a straightforward synthetic pathway that produces non-toxic salt byproducts. This development is pivotal in making the synthesis of sulfonyl fluorides more accessible, cost-effective, and scalable, addressing some of the pressing needs in the field of green chemistry.
With the potential for broad applicability, this new synthetic protocol may significantly impact both academic research and industrial applications. The ability to generate sulfonyl fluorides containing a variety of structural motifs—ranging from aromatic to heterocyclic groups—further enhances the versatility of this method. It aligns well with the ongoing shift toward environmentally conscious practices in chemistry, emphasizing the need for attachment of value to how chemical reactions are designed and executed.
Corresponding authors Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem aptly point out that the development of green synthetic protocols plays a critical role in achieving the Sustainable Development Goals (SDGs). As societies become increasingly aware of the environmental ramifications of chemical processes, integrating sustainability into synthetic practices will undoubtedly become a prominent theme in research agendas.
Ultimately, the synthesis of sulfonyl fluorides through this safe and efficient methodology not only represents a milestone in click chemistry but also underscores a broader commitment to sustainability in the chemical sciences. As this research gains traction, it could redefine conventional practices in chemical synthesis, paving the way for greener alternatives that benefit both researchers and the environment. The continuing exploration of such innovative methods is essential in addressing the urgent challenges of today’s chemical landscape while contributing positively to global sustainability goals.