Recent research from the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig has unveiled a groundbreaking finding: the gas-phase existence of sulfurous acid (H2SO3) under atmospheric conditions. Published in the esteemed journal Angewandte Chemie, this revelation is particularly remarkable considering the long-held belief in scientific circles that sulfurous acid was elusive and difficult to identify as an isolated molecular entity. In contrast to the more commonly known sulfuric acid (H2SO4), which has been widely studied, the chemical behavior and properties of H2SO3 had remained largely theoretical and speculative until now.
Textbook references suggested that H2SO3 could form in aqueous solutions of sulfur dioxide (SO2); however, concrete evidence remained elusive. Previous experimental efforts primarily identified its corresponding bases, bisulfite (HSO3−) and sulfite (SO3^2−), as products, leaving sulfurous acid itself an academic enigma. The only reported detection of H2SO3 prior to this study occurred in a mass spectrometry experiment in 1988, conducted by Helmut Schwarz’s team at TU Berlin, although that detection lasted merely 10 microseconds. The current study’s results, therefore, stand as a significant advancement in atmospheric chemistry.
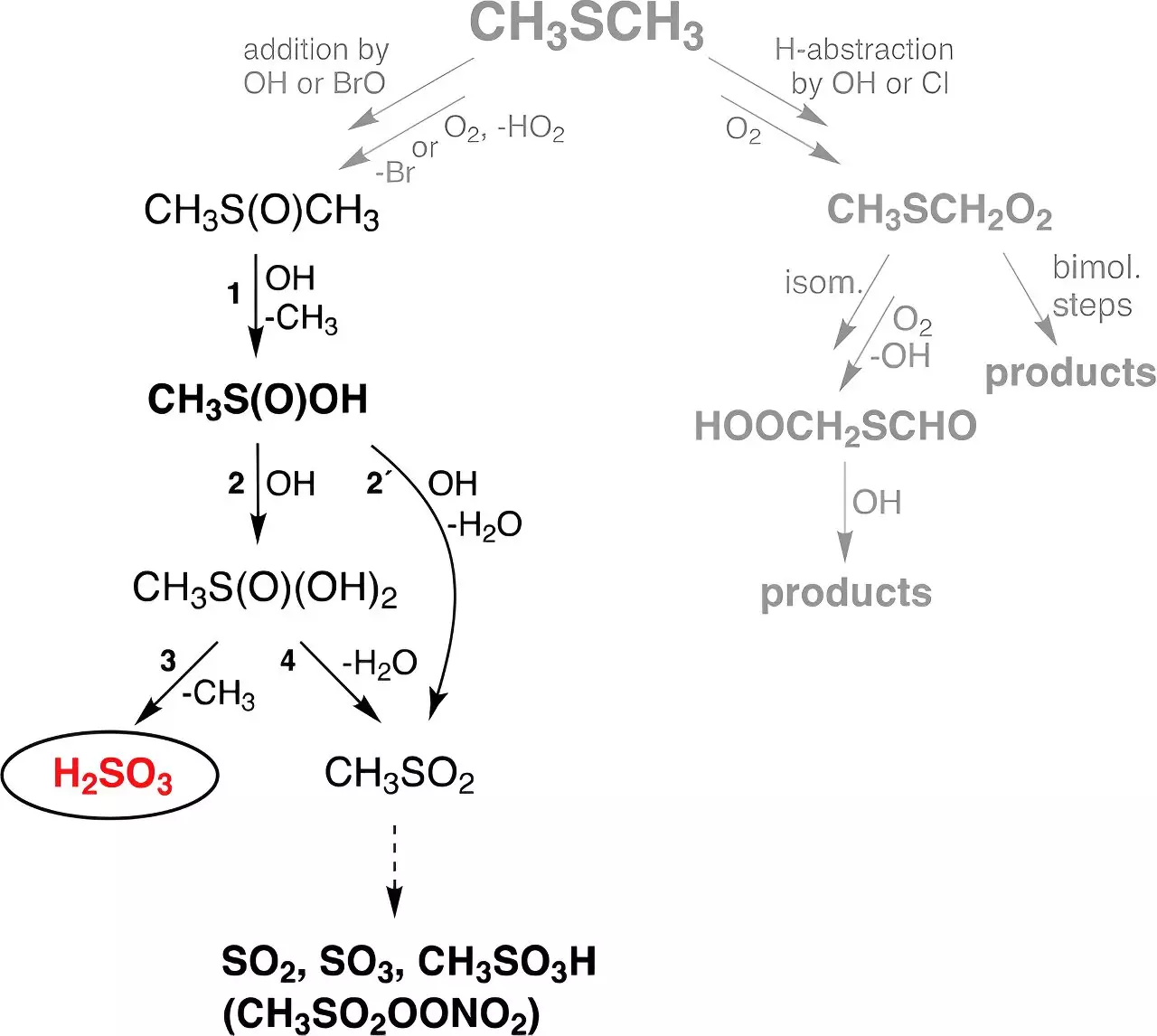
The Role of Dimethyl Sulfide in Formation
The novel research conducted at TROPOS examined the formation of H2SO3 through reactions involving dimethyl sulfide (DMS), a compound primarily produced by marine biogenic processes. DMS contributes approximately 30 million tons of sulfur to the atmosphere annually, thereby serving as an important player in the atmospheric sulfur cycle. Researchers focused on elucidating the theoretical calculations that suggested the potential formation pathway of H2SO3 from the reaction of hydroxyl (OH) radicals—produced by ozone and water molecules under ultraviolet (UV) radiation—with DMS.
To enhance the understanding of atmospheric chemistry, experimental conditions replicated the requisite frameworks in flow reactors, demonstrating the stability of sulfurous acid in gas-phase conditions. Fascinatingly, H2SO3 could remain stable for up to thirty seconds under varying humidity levels, suggesting that it may possess a longer lifetime in the atmosphere than previously acknowledged.
The implications of this discovery extend beyond mere laboratory findings. The researchers at TROPOS integrated their results into an established global chemistry-climate model, leading to estimations that roughly 8 million tons of H2SO3 are being generated globally each year. This amount significantly exceeds previous expectations, producing nearly 200 times more mass of H2SO3 from DMS reactions compared to the direct atmospheric formation of sulfuric acid. This conclusion could alter how scientists perceive and model the atmospheric sulfur cycle, enhancing the understanding of sulfur’s role in climate dynamics.
While the detection of H2SO3 marks a triumphant moment in atmospheric chemistry, the study leaves lingering questions regarding the compound’s stability over extended periods in the atmosphere and its reactivity with trace gases. The specific interactions and degradation pathways concerning water vapor and other atmospheric components require further investigation. Dr. Torsten Berndt, a lead researcher on the project, emphasized the need for optimized experiments to delve deeper into these aspects, consolidating the importance of advanced research methodologies in chemistry.
This newfound capability to detect H2SO3 in atmospheric conditions is indicative of a broader trend of discovering novel reaction pathways and verifying the existence of previously theoretical compounds. The combination of enhanced detection methods—such as an advanced mass spectrometer capable of detecting minute quantities of molecules—and refined experimental controls has proven crucial for these accomplishments.
The identification of sulfurous acid as a gas-phase species under atmospheric conditions encapsulates a paradigm shift in our understanding of atmospheric chemistry. This research not only enriches the existing knowledge of sulfur compounds but also raises profound questions about chemical processes in the environment. As scientific knowledge advances, it is imperative to continue investigating the implications of such discoveries, as they could have enduring impacts on climate modeling, environmental science, and our overall comprehension of atmospheric dynamics. The evolving landscape of chemical research highlights the potential for further breakthroughs that could redefine our understanding of the world around us.