The presence of ammonia (NH3) in modern food production and industrial applications is inarguably significant. With the ammonia market valued at approximately $67 billion and producing around 175 million metric tons globally, its role is as essential as it is multifaceted. The burgeoning hydrogen economy has also earmarked ammonia as a potential high-energy-density carrier, underscoring its critical importance. Despite its advantages, the traditional method of producing ammonia, primarily through the energy-intensive Haber-Bosch process, poses considerable environmental threats due to its high CO2 emissions. Recent advancements, however, have opened doors to more sustainable alternatives, particularly through the electrochemical conversion of nitrate to ammonia.
A research group led by Hao Li from Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) has made remarkable strides in this domain. The team’s study, published in “Advanced Science” on August 9, 2024, explores nitrate reduction (NO3RR) as an innovative method for ammonia production. This process presents a compelling alternative to nitrogen reduction reaction (NRR); while the latter requires breaking the robust N≡N triple bond found in nitrogen gas, nitrate offers a less energy-intensive path due to its lower dissociation energy and higher water solubility. This shift holds significant importance not only for industrial efficiency but also for addressing environmental issues related to nitrate accumulation in aquatic systems.
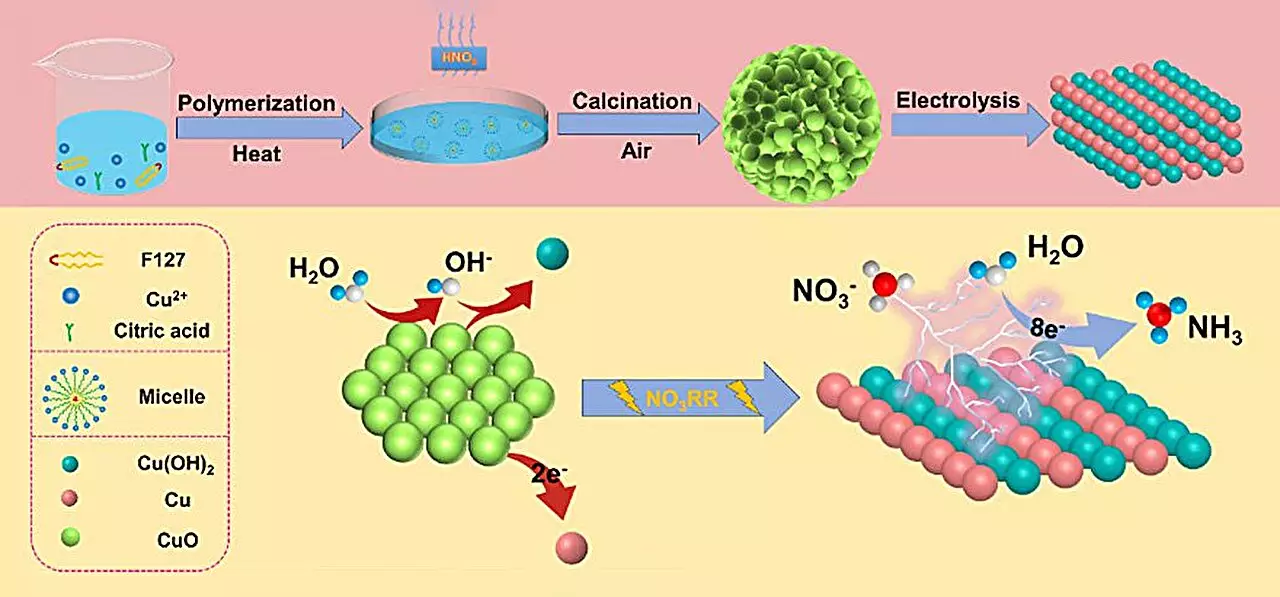
At the heart of this innovative approach lies the use of a specialized copper (II) oxide (CuO) catalyst developed by Li’s team. This spherical catalyst, characterized by its oxygen-rich vacancies and small particle stacking, has demonstrated a remarkable ammonia yield of 15.53 mg h⁻¹ mgcat⁻¹ and a Faraday efficiency of 90.69% while operating under neutral electrolyte conditions at -0.80 V. Such impressive results indicate that not only does the catalyst facilitate a profound amount of ammonia production, but it also does so efficiently.
The efficiency of this electrochemical system can be largely attributed to the noteworthy transformations the CuO catalyst undergoes during nitrate reduction. A critical insight from co-author Qiuling Jiang indicates that the transition from CuO to a Cu/Cu(OH)2 structure enhances catalytic performance by increasing the number of active sites and improving electron transfer on the electrode surface. This transformation is vital for optimizing the overall efficiency of the nitrate reduction process.
Understanding the Catalytic Mechanism
To further dissect these intricate interactions at play, the researchers employed density functional theory (DFT) calculations to elucidate the catalytic mechanism. These calculations revealed that the formation of Cu(OH)2 considerably lowers the energy barrier for nitrate adsorption, rendering the process more energetically favorable. Moreover, the Cu(OH)2 phase effectively curbs the competing hydrogen evolution reaction, which is notorious for hampering ammonia production efficiency. Interestingly, the presence of Cu (111) crystal surfaces was found to enhance the hydrogenation process, adding another layer of distinction to the catalyst’s functionality.
The implications of Li’s research go beyond local solutions for ammonia production; they signal a shift towards a more sustainable future. By focusing on the controllability of reaction conditions and understanding phase transitions in catalysts, it may soon be possible to create catalysts that are not only high-performing but also scalable for broader industrial applications. Future research is expected to delve deeper into the factors that affect these phase transitions, aiming to optimize not just the efficiency, but also the sustainability of ammonia production—an essential factor in addressing global food security and industrial requirements.
The research conducted by Li and his team highlights a significant step towards transforming the landscape of ammonia synthesis. By leveraging the electrochemical reduction of nitrate, they present a pathway that mitigates environmental concerns while promising enhanced efficiency. This pioneering work opens the door to a sustainable future in ammonia production, bearing implications that extend well beyond the laboratory to industries poised for change.