The exploration of lipids, particularly sphingolipids, has undergone significant evolution since the late 19th century. Renowned pathologist Ludwig Thudichum’s pioneering work involved isolating previously unknown fatty substances from the brain, which he termed sphingolipids. This nomenclature was inspired by the Greek Sphinx, an homage to the enigma that these molecules pose to scientists. Over the decades, substantial evidence has surfaced linking sphingolipid metabolism irregularities to a variety of diseases, notably Fabry’s and Gaucher’s diseases that primarily affect neurological function. However, this is just the tip of the iceberg, as recent investigations have established connections between sphingolipids and infectious diseases, including those caused by viruses such as Ebola and COVID-19, as well as bacterial pathogens like Pseudomonas aeruginosa.
Central to this discussion is sphingomyelin, a prominent sphingolipid whose metabolism is critical in a host of pathological conditions. Key to many infections is the enzyme sphingomyelinase, which facilitates the breakdown of sphingomyelin. The degradation of this molecule is orchestrated during infections, where pathogens exploit or manipulate host lipid metabolism for their survival and replication. Up until now, researchers faced a significant hurdle: the inability to visualize the activity of sphingomyelinase during pathogen interactions. The implications of this limitation are enormous, as understanding these biological processes at the molecular level is essential for developing effective therapeutic strategies.
Researchers from the Universities of Wurzburg and Berlin have made headway in overcoming this challenge by designing a novel sphingomyelin derivative that enables the visualization of sphingomyelin distribution and sphingomyelinase activity. Their findings, recently published in *Nature Communications*, open a new avenue for exploring the interaction between sphingomyelin metabolism and infections. The researchers, part of the Research Training Group 2581, a collaborative consortium of chemists, physicists, and biologists, synthesized trifunctional sphingomyelins—molecules that not only mimic natural sphingomyelin but also possess additional functionalities that render them desirable for research applications.
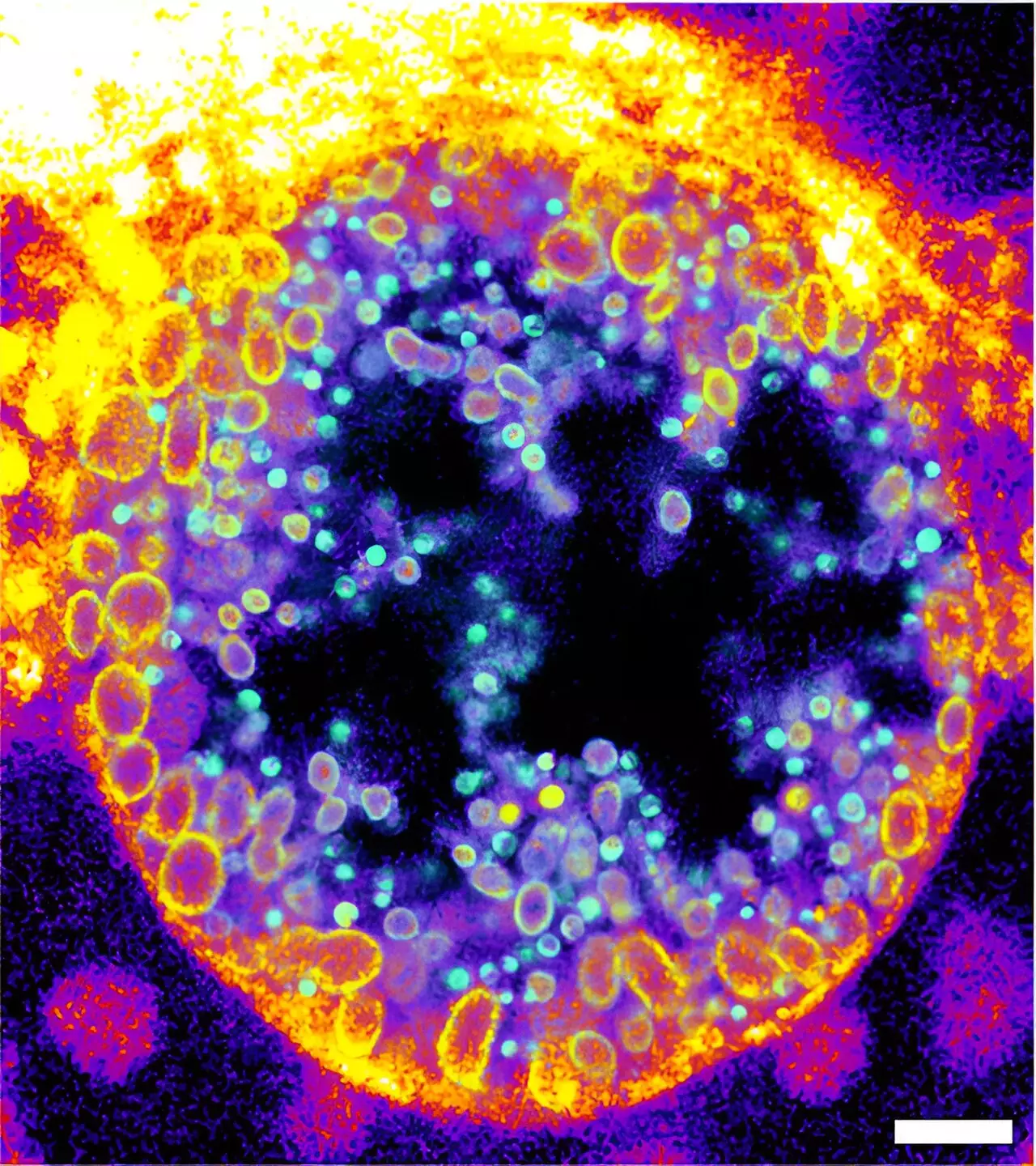
The novel trifunctional sphingomyelins developed serve as a chemical tool to trace sphingomyelin degradation during infections. Professor Jürgen Seibel, a key contributor to this research, emphasized the challenge faced in designing molecules that are metabolically accepted and functionally significant within human cells. The researchers meticulously demonstrated the effects of bacterial sphingomyelinase on human cell surfaces and elucidated the progression of sphingomyelin degradation during Chlamydia infections—a pathogen notorious for its role in sexually transmitted diseases and potential oncogenesis. The implications of this work extend beyond mere observation, as it paves the way for targeted therapeutic interventions by providing real-time monitoring of infection processes.
The innovative method of visualization through expansion microscopy and click-chemistry enabled a detailed analysis of sphingomyelin’s metabolism within Chlamydia-infected cells. The research uncovering that chlamydial inclusions are significantly composed of cleaved sphingomyelin forms signals that sphingomyelin degradation plays a decisive role in the lifecycle of these pathogens. Understanding these mechanisms is vital, not only for elucidating the pathogenesis of Chlamydia but also for the broader realm of infectious disease research, offering prospects for developing new treatment strategies that can halt pathogen progression at the metabolic level.
The development of this new sphingomyelin derivative marks a significant milestone in infection research. As Professor Seibel concludes, the adaptability and functionality of these chemical tools hold promise for widespread applicability in various laboratory settings. This advancement not only fosters a deeper comprehension of sphingomyelin metabolism in infection dynamics but also propels the field toward innovative approaches for combating infectious diseases. The enthusiasm surrounding these findings reflects a pivotal moment in lipid research, demonstrating how an understanding of molecular interactions can lead to significant breakthroughs in health science.