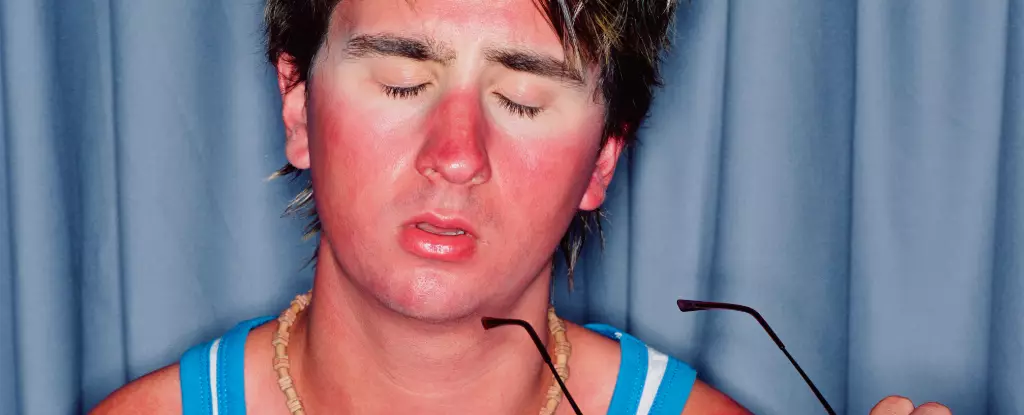
For decades, the understanding of sunburn has been largely simplistic, reducing it to a painful reaction stemming primarily from DNA damage. The established narrative claims that exposure to harmful ultraviolet (UV) rays, particularly UVB, leads to a series of biochemical events that ultimately result in the inflammation of the skin, commonly referred to as sunburn. However, recent findings from a study led by molecular biologist Anna Constance Vind at the University of Copenhagen suggest that there is more to the story. The crux of this new research challenges conventional wisdom, positing that it is the damage to RNA rather than DNA that triggers the immediate inflammatory response during sunburn.
This revelation is not merely an academic footnote; it has profound implications for our understanding of dermatological health and skin reactions to sun exposure. The potential for RNA damage to signal distress in skin cells complicates the narrative around sunburn and may pave the way for new treatment avenues that focus on preventing initial responses to UV exposure rather than merely addressing aftereffects.
Traditional models explain sunburn as a result of thermal burns, where heat alters the body’s proteins. Yet, the mechanism of sunburn is distinctly different. Sunburn arises from prolonged exposure to UVB radiation, a type of energy that is uniquely destructive to skin cells. Such exposure does more than simply raise the temperature of the skin; it creates a complex suite of cellular stresses that activate the immune system, prompting a cascade of responses that include swelling, redness, and pain.
Initially, it was thought that the nucleic acids in our DNA would become damaged by UV photons, resulting in mutations that might propagate cellular malfunctions. This DNA-centric viewpoint has dominated textbooks and research for years. However, Vind’s team has redirected focus toward RNA, specifically how the damage incurred at this level might be a pivotal early signal that spurs the body into action. Such a fundamental shift in our understanding is not only enlightening but also suggests that protective measures might need to be re-evaluated.
Vind’s research employed a range of innovative techniques, including genetically modified mice missing a stress response protein known as ZAK-alpha. This protein has a critical role in alerting cells to stressors during the translation of messenger RNA into proteins. By exposing these mice to UVB radiation and observing their reactions, researchers discovered that the absence of ZAK-alpha inhibited the typical signs of sunburn. This suggests that the messenger RNA, previously considered secondary in importance, may be the first line of defense in the skin’s response to UV damage.
Opening a dialogue about RNA’s role could reshape preventive strategies for sunburn. If RNA acts as a more immediate alert mechanism, it may also guide the development of therapies that reinforce this cellular response. Moreover, this newfound understanding could lead dermatologists and skincare experts to reconsider product formulations, focusing more on protecting RNA integrity rather than only on DNA preservation.
The implication of Vind’s findings is twofold: while DNA damage remains a critical concern for long-term cellular health, the immediate responses to sun exposure might be more readily influenced by safeguarding RNA. With further investigation into how RNA functions under UV stress, there might be potential for preventative treatments that amplify the body’s natural defenses.
As societies increasingly recognize the importance of sun protection—given the rising rates of skin cancers and other conditions—research that reveals previously overlooked mechanisms can be core to public health initiatives. Skincare products that can stabilize messenger RNA during UV exposure may become vital components in sun care regimes. Moreover, public health messaging might also evolve to inform individuals about the importance of not merely shielding against UV rays but also understanding the cellular processes at play.
As we grapple with our relationship with the sun, Vind’s groundbreaking research represents a pivotal moment in dermatological science. By unraveling the sequence of cellular events that lead to the painful, albeit familiar experience of sunburn, this work highlights the need for an expansive understanding of skin responses to UV radiation. This shift not only aids our comprehension of how sunburn manifests but also heralds new opportunities for developing effective treatments. Ultimately, embracing a holistic view that considers both DNA and RNA damage promises to enhance skin health and protection against the sun’s ever-present rays.