Z-alkenes are an intriguing class of organic compounds characterized by a carbon-carbon double bond, with substituents located on the same side of that double bond. This unique configuration makes them essential in various chemical and biological processes. Their significance cannot be overstated; they serve as critical structural elements in countless organic reactions and pharmaceutical compounds.
Despite their importance, Z-alkenes pose significant synthetic challenges. Traditional routes often yield poor results, creating hurdles for chemists aiming to harness their full potential. However, innovative methods are emerging, illuminating new pathways for the efficient synthesis of Z-alkenes, particularly through advanced methodologies like photoisomerization.
Photoisomerization stands out as a transformative technique in organic chemistry. Focusing on the conversion of E-alkenes to Z-alkenes, this method utilizes the power of light to change molecular structures, thus offering a practical approach to producing these compounds. An array of applications spurs from this process, spanning organic chemistry, polymer science, and even medicinal chemistry.
Recent investigations have underscored challenges in existing photoisomerization techniques, particularly those involving ionic liquids. While ionic liquids have shown promise due to their unique properties, the methods are often cumbersome, requiring intricate setups that can hinder their practical application in high-performance liquid chromatography (HPLC). The necessity for alternatives to streamline these processes has never been more pronounced.
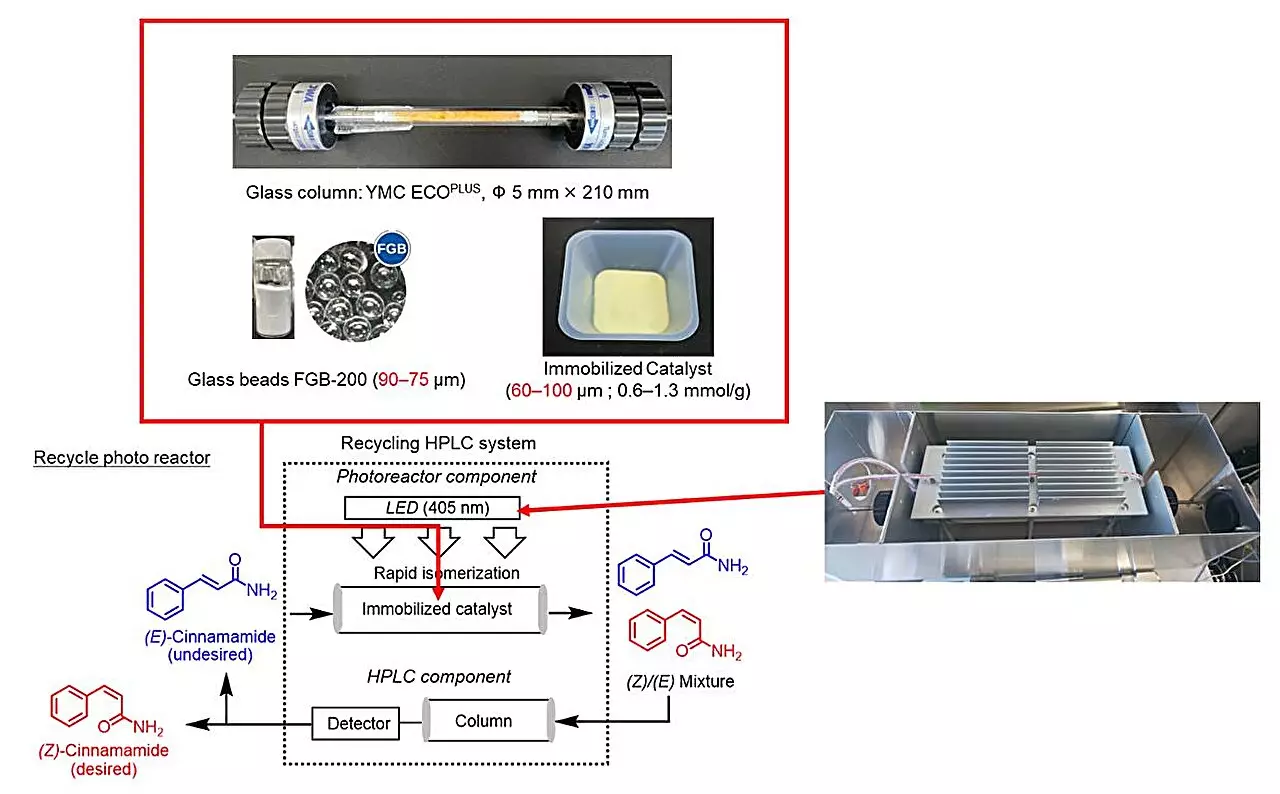
A recent study spearheaded by Professor Hideyo Takahashi and his team at the Tokyo University of Science has provided a groundbreaking solution. This research specifically targeted the photoisomerization of E-cinnamamides—an essential precursor to various Z-cinnamamides—using a recycling photoreactor intertwined with HPLC technology. The closed-loop system they developed bifurcates the process: it separates the desired product while continuously regenerating the catalyst, thereby maximizing efficiency.
Prof. Takahashi’s approach builds on previous innovations in deracemization, taking the principles of racemic mixture conversion and applying them to alkenes. The adaptability of the closed-loop system signifies a paradigm shift in organic synthesis, highlighting its potential to create eco-friendly protocols that respect both scientific advancement and environmental stewardship.
Integral to the photoisomerization process are photosensitizers—substances that amplify the reaction rates through light absorption. The study identified thioxanthone as the premier candidate due to its ability to accelerate the isomerization between E- and Z-cinnamamides effectively. What sets thioxanthone apart is its functional groups, which serve a dual purpose: anchoring it securely in the solid phase while enhancing its catalytic activity.
Such enhancements challenge the traditional narrative that solid-phase reactions generally progress more slowly than their liquid-phase counterparts. This shift in understanding ignites new possibilities for future research while confirming the transformative impact of molecular engineering on catalyst design.
The implications of Takahashi’s work extend far beyond mere academic interest. By introducing a more efficient method to synthesize Z-alkenes sustainably, this research paves the way for greener pharmaceuticals and encourages the development of processes that have a lower environmental impact. The closed-loop recycling photoreactor not only exemplifies innovation but also embodies a commitment to sustainability in chemical manufacturing.
As the organic chemistry community grapples with increasing demands for sustainable practices, Takahashi’s method represents not just an efficient pathway for Z-alkenes but also a paradigm for other synthetic processes. The intricate dance between chemistry and environmental responsibility, once fraught with discord, now has the potential to achieve harmony through innovative approaches and thoughtful design.
The evolution of organic synthesis showcased in this study underscores the importance of merging science with sustainability, revealing that the future of chemistry is not merely about advancing knowledge but also about addressing the ethical implications and responsibilities that come with it. As we venture into this new frontier, let us embrace the synergy of innovation and sustainability, guiding us toward a brighter, greener future.