The quest for sustainable energy solutions continues to inspire groundbreaking research and innovation. A recent study led by Professor Jeong Woo Han and his dedicated team from Pohang University of Science and Technology (POSTECH) and Seoul National University has unveiled a catalyst that promises to revolutionize hydrogen production in alkaline water electrolysis systems. This remarkable development not only addresses the persistent issue of degradation caused by reverse current but also enhances the overall efficiency of hydrogen generation, crucial for advancing our renewable energy efforts.
The Challenge of Energy Fluctuations
As society shifts towards a more sustainable future, the reliance on renewable energy sources—such as solar, wind, and hydro—has become increasingly significant. These sources, however, are inherently unpredictable; their output varies with changing weather conditions and times of day. Storing and effectively utilizing this energy is paramount, and hydrogen emerges as an excellent candidate due to its high energy density and versatility. The challenge lies in producing hydrogen efficiently and reliably, particularly through water electrolysis, where electricity is used to split water into hydrogen and oxygen.
Understanding the Alkaline Water Electrolysis System
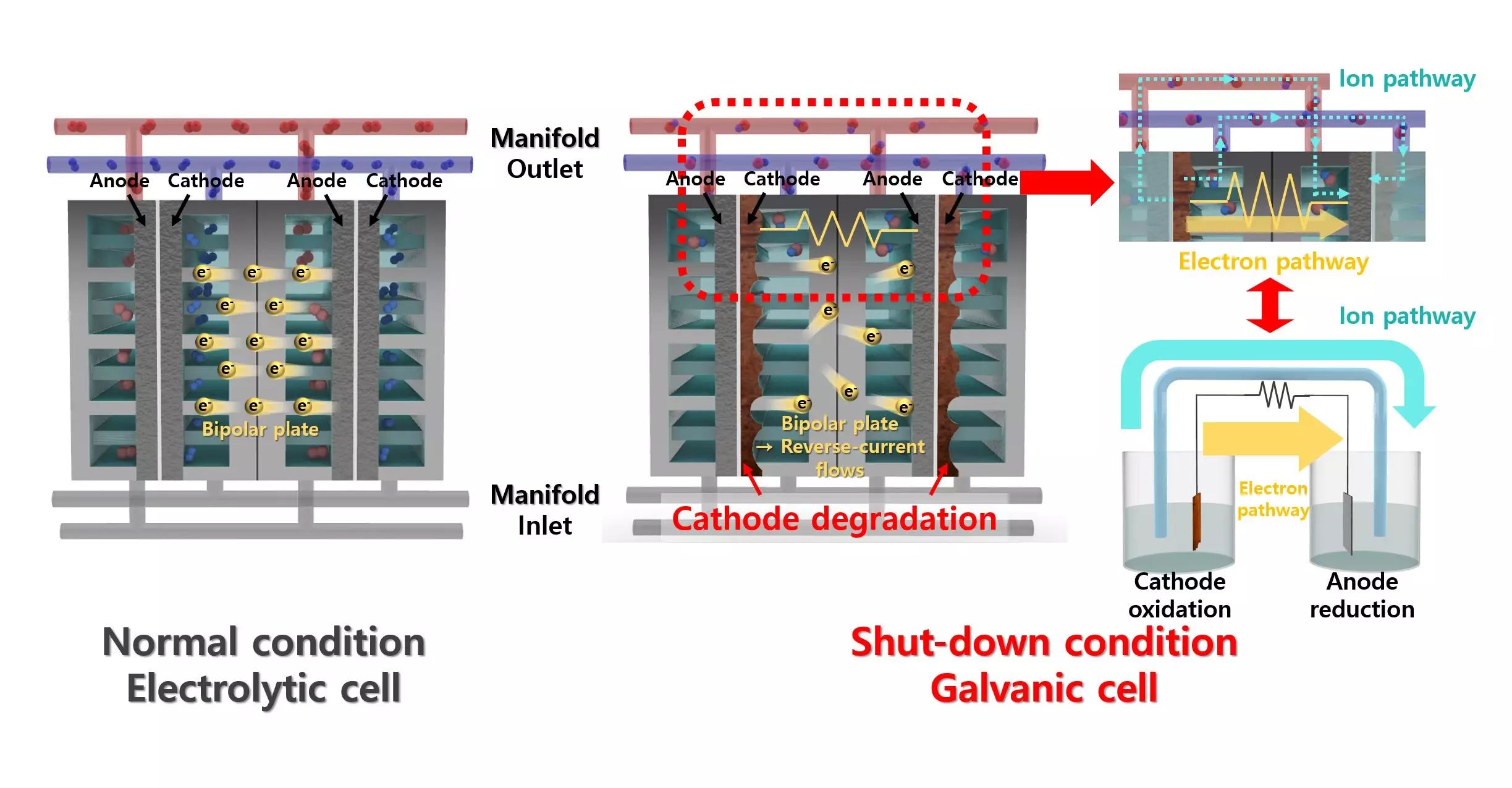
Alkaline water electrolysis (AWE) presents a unique solution to hydrogen production, offering an economically viable and durable method of generation. While it has notable advantages, including lower operating costs, the intermittent nature of renewable energy adds complexity to the AWE process. When the power supply is inconsistent, reverse currents can develop, potentially damaging the catalyst materials and reducing their longevity. This poses a significant drawback in the long-term application of AWE technology.
Introducing the Innovative Catalyst
In an astute response to these complications, the research team developed a catalyst that employs a lead (Pb) coating atop a nickel (Ni) catalyst. While lead is not typically favored in hydrogen evolution contexts due to its poor catalytic activity, this innovative approach leverages lead as a co-catalyst. It effectively enhances the catalyst’s performance by promoting essential reactions such as proton desorption and water dissociation. This creative blend not only mitigates catalyst degradation but also maximizes efficiency during the hydrogen evolution reaction.
Significance of the Study
The implications of this study are vast. With the introduction of a simple lead coating, the researchers have demonstrated a compelling solution to the issue of reverse current without the need for additional equipment, which has historically complicated AWE systems. As noted by Professor Yong-Tae Kim, this research represents a pioneering step towards solving degradation issues in alkaline water electrolyzers, establishing a foundation for further exploration in this critical arena of renewable energy technology.
In essence, this catalyst breakthrough signifies a monumental leap in hydrogen production capabilities. As researchers continue to refine this technology, the potential for wider application in sustainable energy systems appears more promising than ever. The future may very well hinge on advancements like these that effectively address existing inefficiencies and foster the transition to a hydrogen-powered society.