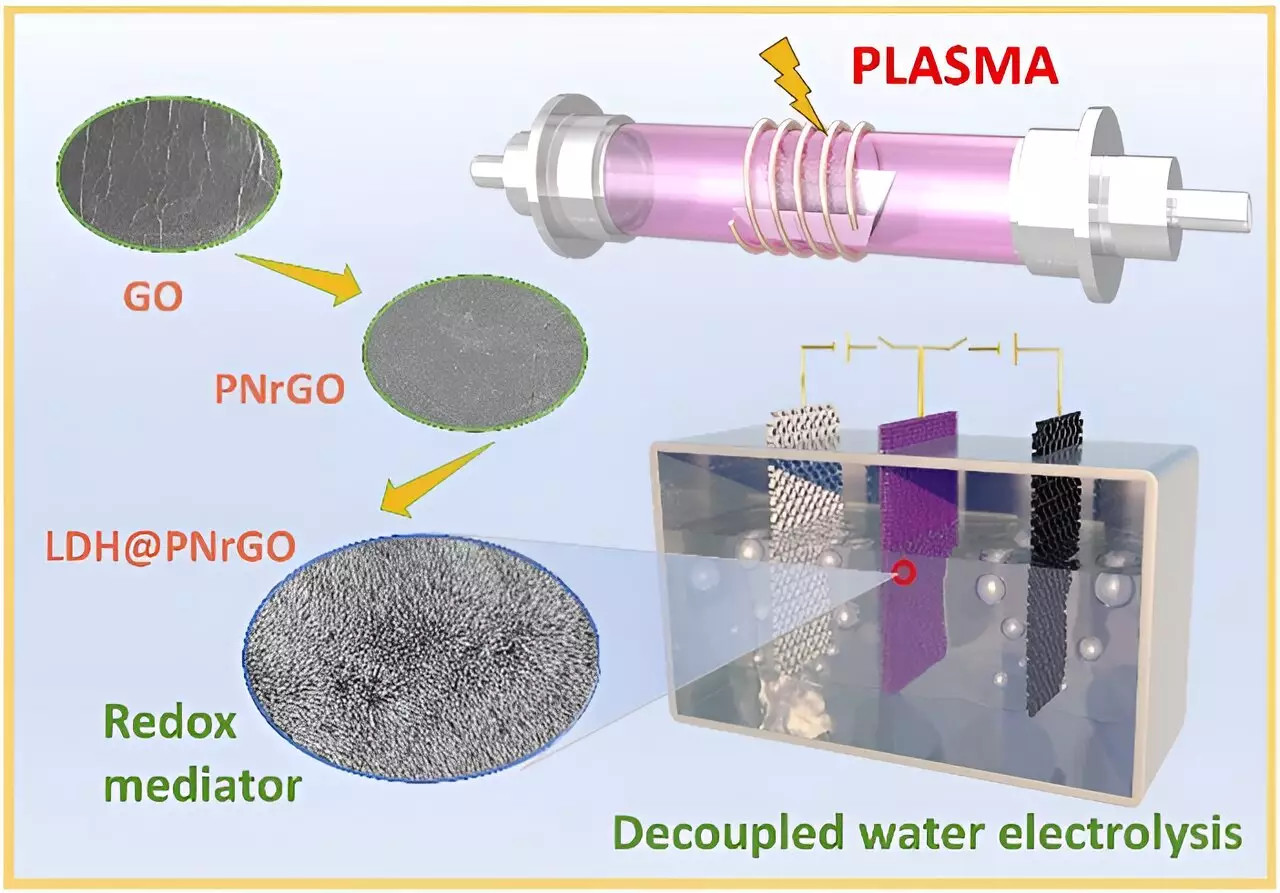
The quest for efficient hydrogen production has long been entangled with the limitations of traditional alkaline electrolyzers. Despite their promise for renewable energy integration, these systems struggle with a variety of issues, specifically around fluctuating energy sources and the risk of hydrogen and oxygen mixing at high pressures. German physicist Heinrich Hertz once said, “One must be a man of the world to understand the world.” In this context, it is clear that a more nuanced understanding of the electrolysis process is essential for overcoming these obstacles. A recent study conducted by a team led by Professor Chen Changlun at the Hefei Institutes of Physical Science represents a pivotal shift in our approach to water electrolysis.
Innovations in Two-Step Water Electrolysis
The researchers developed cobalt-doped nickel hydroxide bipolar electrodes that address the inherent limitations of conventional nickel hydroxide. This innovation is significant because it not only enhances electrochemical performance but radically transforms the mechanism through which hydrogen and oxygen are produced. By employing a two-step process—thus separating the production phases in both time and space—this groundbreaking approach eliminates the need for costly membrane separators, further streamlining the system’s efficiency.
Cobalt doping plays a critical role in this advancement, as it boosts the conductivity and energy storage potential of nickel hydroxide electrodes. The resultant increase in resilience against parasitic reactions means that hydrogen production becomes more straightforward and efficient, shedding light on pathways to sustainable energy solutions.
The Role of Non-Noble Metal Catalysts
In further bolstering the efficiency of this technology, the research team introduced innovative non-noble metal catalysts, such as molybdenum-doped nickel-cobalt phosphide. Their method transcends traditional electrolysis techniques by enhancing durability and catalytic activity while maintaining low operational voltages. What sets this research apart is not merely the performance indicators but the intent to make hydrogen production more accessible and less reliant on precious metals, which typically inflate operational costs and limit scalability.
The coupling of bipolar electrodes with cutting-edge catalysts allows for dynamic switching of current, facilitating independent hydrogen and oxygen production. This represents a crucial advancement for populations seeking sustainable energy solutions without the burdensome costs associated with conventional methods.
Future Implications and Industrial Applications
The findings published in prestigious journals indicate that the new two-step water electrolysis method could dramatically influence large-scale hydrogen production and storage, with implications extending to various sectors, including telecommunications and energy infrastructure, like 5G base stations and data centers. By embracing these innovations, the industry can not only meet global performance standards but may exceed them, pushing towards an era where hydrogen becomes an invaluable player in clean energy economies.
Professor Chen Changlun’s sentiment regarding the alignment of their performance indicators with global benchmarks underscores the relevance this research holds for future industrial operations. As we stand at a crossroads in energy production, breakthroughs like these offer glimmers of hope in creating a more efficient, economical, and sustainable hydrogen economy. As advancements in technology continue to unfold, the chemical engineering field stands poised for a significant transformation that could fundamentally shift our approach to energy consumption and production.