The marvel of gallium lies not only in its unique physical characteristics but also in its enigmatic atomic behavior. Nearly 150 years after its discovery by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, a recent study conducted by scientists from the University of Auckland has illuminated previously unrecognized dimensions of this intriguing element. As it exists in the periodic table, gallium’s low melting point allows a gallium spoon to melt away in a steaming cup of tea. This peculiarity has captivated researchers for decades, but the fresh findings provide critical insights that could revolutionize our understanding of gallium’s applications, particularly in the realm of nanotechnology.
Gallium’s Unconventional Behavior
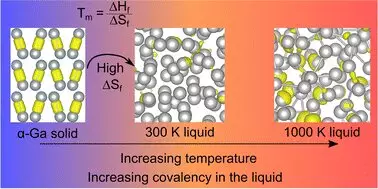
Gallium challenges traditional perceptions of metals through its behavior at the atomic level. Distinct from most metallic elements, gallium predominantly exists as ‘dimers,’ which are pairs of atoms bonded together. This unusual arrangement equips gallium with properties that defy the norm, such as being less dense when solid than when liquid—an anomaly reminiscent of ice floating on water. Typically, metallic bonds prevail in elemental forms, but gallium showcases covalent bonds, a feature usually associated with non-metals, where atoms share electrons rather than engaging in the more familiar metallic bonding.
The latest research introduces a profound shift in the understanding of gallium’s molecular structure. In the groundbreaking paper titled “Resolving Decades of Debate: The Surprising Role of High-Temperature Covalency in the Structure of Liquid Gallium,” published in *Materials Horizons,* the authors provide compelling evidence that gallium’s covalent bonds diminish at melting point but intriguingly re-emerge at elevated temperatures. This revelation directly contradicts decades of established assumptions and demands a fresh explanation for why gallium exhibits such a low melting point compared to other metals.
A Paradigm Shift in Scientific Understanding
Professor Nicola Gaston, who spearheaded the research alongside Dr. Steph Lambie and Dr. Krista Steenbergen, emphasizes the monumental shift in scientific discourse regarding gallium. For over thirty years, the prevailing scientific literature has been based on an incorrect foundational assumption concerning gallium’s structure. This re-evaluation effectively challenges the collective wisdom, opening pathways to insights that were previously obscured. Gaston’s statement illustrates the need for continued inquiry and the reevaluation of accepted norms in science: “Thirty years of literature on the structure of liquid gallium has had a fundamental assumption that is evidently not true.”
At the heart of this new understanding lies the concept of entropy—the measure of disorder within a system. The team proposes that the significant increase in entropy when gallium bonds break plays a pivotal role in allowing the metal’s atoms to move freely, thus facilitating its liquidity states and unique physical properties. This link between atomic dynamics and thermodynamic principles stands as a testimonial to the intricate balance inherent within material science.
Technological Implications and Future Prospects
As technological giants endeavor to manipulate materials at the nanoscale, understanding gallium’s behavior becomes fundamental to advancements in various applications. From semiconductor technology to innovative liquid metal catalysts and self-assembling structures, gallium plays a crucial role in the evolution of modern materials science. The implications of such research extend far beyond theoretical inquiries, impacting industries from aerospace and defense to telecommunications and renewable energy.
One of the most exciting prospects lies in gallium’s potential to aid in the search for past microbial life on Mars. Researchers within the University of Auckland’s School of Environment are exploring how gallium could function as a chemical fingerprint, preserving biochemical markers left behind by ancient life forms. This intriguing intersection of material science and astrobiology highlights the expansive relevance of gallium beyond terrestrial applications.
The Legacy of Discovery
Gallium’s journey from theoretical prediction to practical application weaves a narrative as rich as the metal itself. Dmitri Mendeleev, credited with the creation of the periodic table, foresaw the existence of gallium long before it was identified, showcasing the prescient nature of scientific inquiry. In calling it gallium—derived from Gaul, indicative of its French discoverer—this metal not only pays homage to its origins but also represents a culmination of intellectual perseverance and curiosity.
Gallium stands as a testament to the power of scientific exploration, serving as a reminder of how the smallest elements can lead to vast revelations. The ongoing investigations into its structure and behavior continue to highlight the profound complexities of the material world, inviting us to delve deeper into the intricacies of nature.