In our quest for environmental sustainability, the challenge of micropollutants—those toxic chemicals, including pesticides and trace substances—remains a pressing concern. Their prevalence in water systems often results from agricultural runoff and industrial discharges, posing serious threats to both ecosystems and human health. The traditional methods of filtration and chemical treatments frequently fall short, highlighting the urgent need for innovative and efficient removal techniques. This is where the concept of photocatalysis shines, harnessing the power of light to transform our ability to neutralize these insidious pollutants effectively.
The Science Behind Photocatalysis: Semiconductors and Nanomaterials
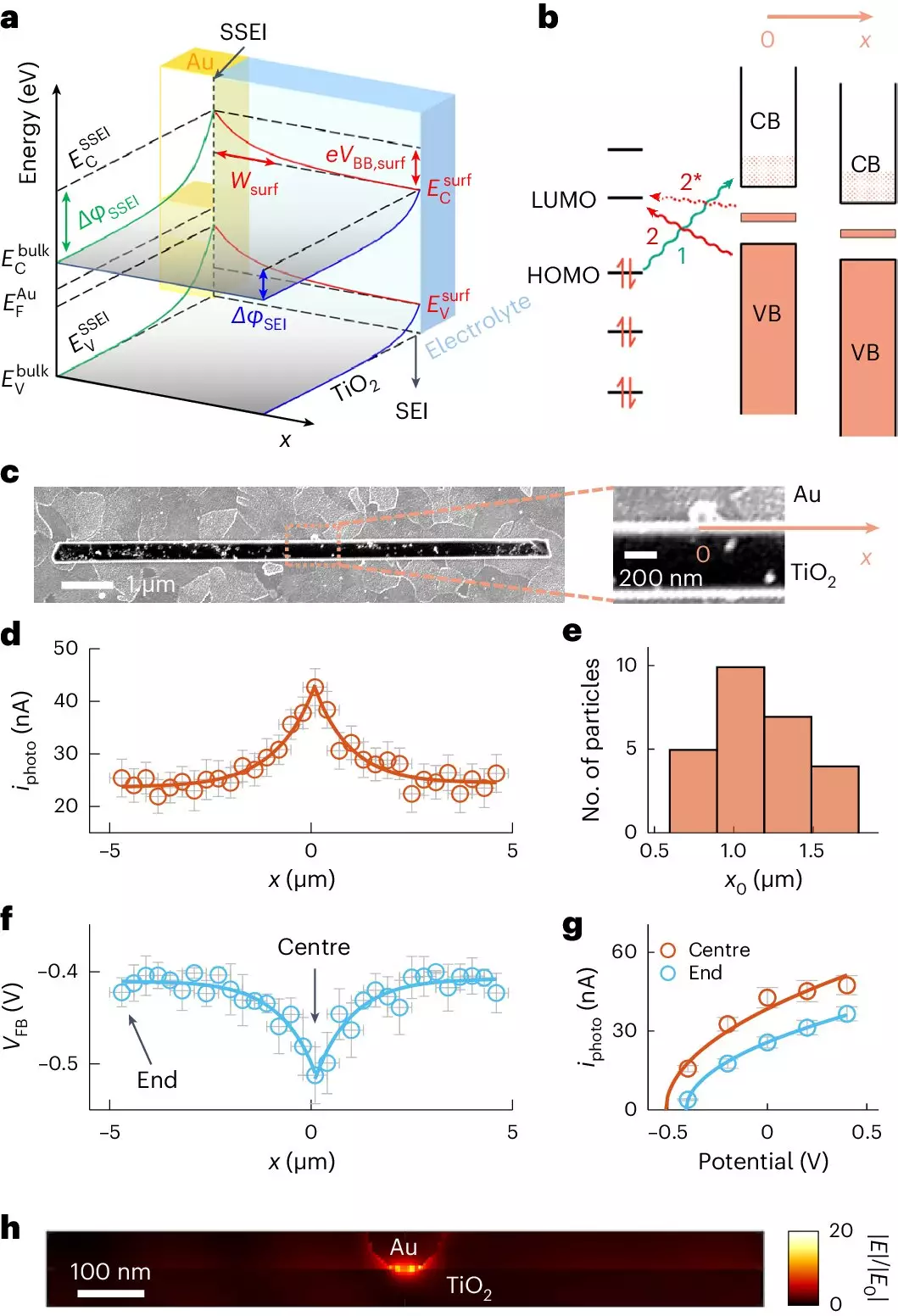
At the heart of photocatalysis lies the interaction between light and semiconducting materials. Titanium dioxide (TiO2) has emerged as a frontrunner in this field due to its stability, non-toxicity, and ability to harness solar energy. However, the effectiveness of TiO2 is significantly influenced by its surface interactions with micropollutants. To enhance these interactions, researchers are increasingly exploring the use of nanomaterials, particularly gold nanoparticles, as co-catalysts. This seemingly simple amendment introduces a novel twist to traditional photocatalytic processes, extending their potential far beyond prior limitations.
Recent Innovations: adCOMPEITS and High-Resolution Imaging
One of the breakthroughs in photocatalytic research is the introduction of a cutting-edge imaging technique known as adCOMPEITS, which stands for Adsorption-based COMPetition Enabled Imaging Technique with Super-resolution. Developed by a research team at Cornell University, this technique enables scientists to visualize and quantify the adsorption phenomena happening at the microscopic level. By employing fluorescent probe molecules that compete with harmful micropollutants for surface attachment, researchers can create detailed maps of adsorption with remarkable clarity.
The implications of this innovation are significant. It allows scientists to assess not only how much of a pollutant can adhere to a surface but also how effectively nanomaterials, such as gold, can facilitate this process. By enhancing our understanding of these dynamics, researchers can fine-tune photocatalytic systems for optimal performance against a variety of pollutants.
Gold Nanoparticles: The Unsung Heroes of Photocatalysis
The most fascinating aspect of the research led by Professor Peng Chen and his team is the revelation of how gold nanoparticles can effectively manipulate surface properties of TiO2. The findings indicate that these nanoparticles can impact adsorption behavior considerably, even at distances well beyond their physical presence, demonstrating their potential in long-range catalytic activity. This phenomenon, referred to as surface band bending, alters the chemical environment, allowing for enhanced interaction with targeted contaminants.
What’s particularly exciting is that the utilization of gold nanoparticles requires only minimal quantities to achieve significant improvements in photocatalytic efficiency. This not only makes the process more cost-effective but also tackles one of the primary challenges in photocatalysis: the typically low conversion efficiencies of solar energy into usable chemical reactions.
Broader Implications for Environmental Applications
The ramifications of this research extend well beyond the treatment of wastewater. The principles governing the enhanced adsorption capabilities of photocatalytic systems open new avenues for applications in environmental sensing, energy generation via dye-sensitized solar cells, and even in the realms of pharmaceuticals and agriculture. The ability to effectively degrade pollutants while leveraging abundant solar energy could transform how industries engage with environmental responsibilities.
As we grapple with the growing problem of contamination and strive towards sustainable solutions, innovations like those being developed at Cornell could serve as vital tools. The incorporation of nanoscale materials in photocatalysis exemplifies how advancements in nanotechnology can fuel breakthrough techniques in environmental remediation.
The discoveries about the enhancement of photocatalytic processes through the integration of gold nanoparticles into TiO2 systems represent a pivotal stride toward efficiently combating micropollutants and ensuring cleaner water supplies. As research continues to evolve, the potential for widespread application looms tantalizingly on the horizon, promising a future where innovative chemistry plays an integral role in preserving our planet. With each advancement, we edge closer to unlocking a cleaner, brighter, and more sustainable world.