The quest for sustainable energy solutions has led scientists to explore innovative methods of harnessing renewable sources. A pivotal challenge in this endeavor is improving the efficiency of the oxygen evolution reaction (OER), a key process in technologies like water splitting and metal-air batteries. A recent study published in *ACS Catalysis* on August 30, 2024, illustrates promising developments in the creation of cost-effective catalysts that could revolutionize energy storage and hydrogen production.
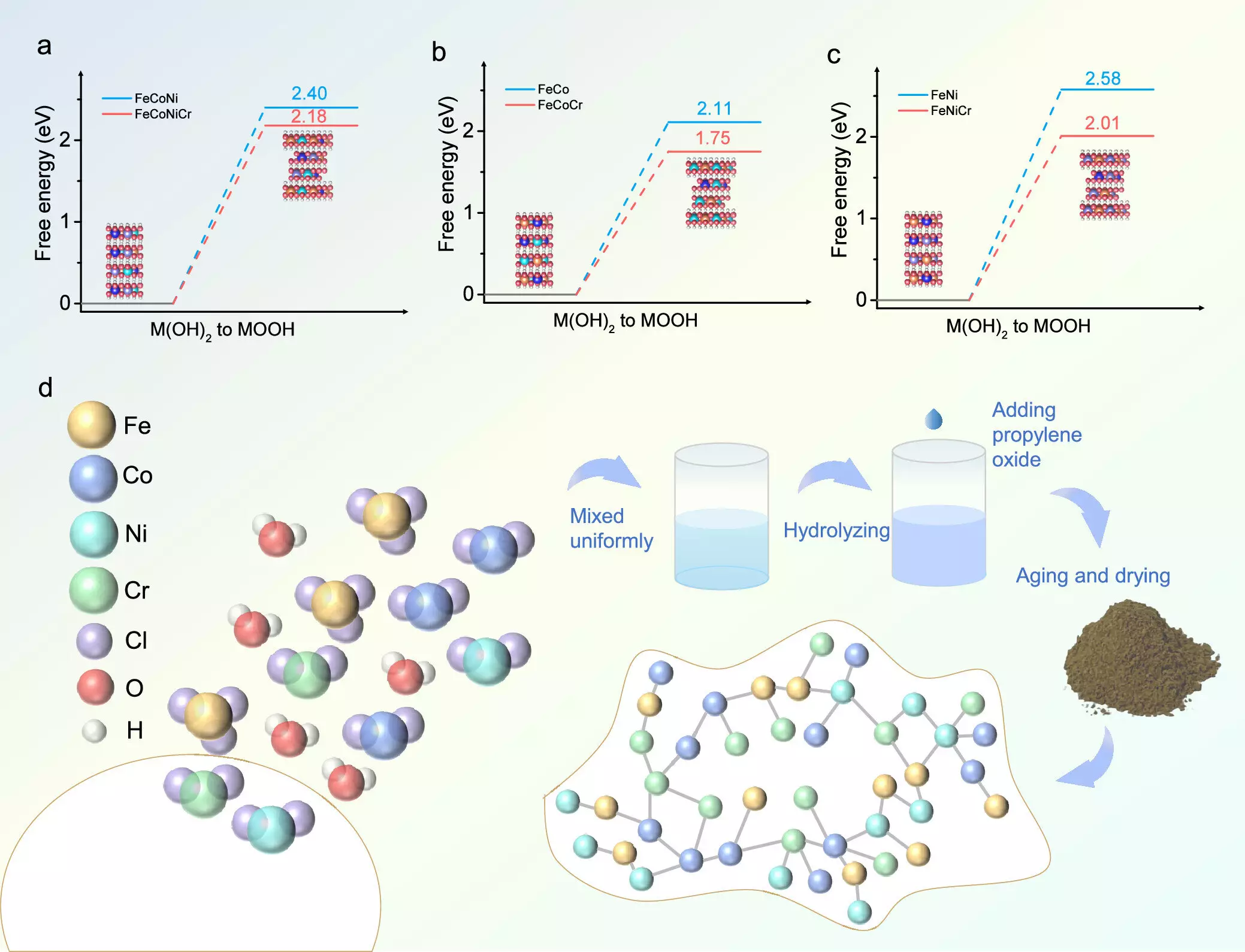
Researchers have achieved notable advances by integrating chromium (Cr) into transition metal hydroxides, facilitating a marked improvement in catalytic activity. By utilizing a combination of density functional theory (DFT) calculations and practical experimental methods, the team developed a new catalyst, FeCoNiCr, through a meticulous aqueous sol-gel technique. This approach ensured that all four metallic elements were evenly distributed, which is crucial for achieving optimal performance.
Hao Li, a key researcher at Tohoku University’s Advanced Institute for Materials Research, emphasized the significance of chromium doping, stating that it enhances the transition of metal hydroxides into the highly reactive oxyhydroxide phase vital for efficient OER. This concept is fundamental as it addresses the slow rates that have hampered past OER efforts, which require highly active and stable catalysts for improvement.
The synthesized FeCoNiCr catalyst demonstrated impressive metrics, achieving a low overpotential of 224 mV in alkaline conditions—exceeding the performance of comparable catalysts by an even greater margin of 52 mV. Furthermore, the catalyst exhibited remarkable stability, remaining effective for over 150 hours. In practical applications, a zinc-air battery utilizing this catalyst maintained consistent operation for 160 hours, demonstrating a low voltage difference of 0.70 V during charge and discharge cycles.
These metrics not only signify enhanced efficiency but also showcase the catalyst’s potential applicability in real-world energy systems, providing a viable solution to the challenge of intermittent renewable energy storage.
Theoretical analyses yielded critical insights into the catalyst’s performance, revealing how chromium doping fine-tunes the adsorption energies of OER intermediates at active sites. This adjustment leads to smoother reaction pathways and thereby accelerates the overall reaction efficiency. Bader charge analysis further revealed that nickel and cobalt remain in an optimal +3 oxidation state during the OER, which is advantageous for maintaining catalytic activity over extended periods.
The findings underscore the interplay between theoretical and experimental approaches in developing catalysts, highlighting their combined efficacy in achieving substantial improvements in OER.
With these promising results in hand, the research group intends to broaden their investigation into other enhancements that could further refine catalyst performance. Di Zhang, an assistant professor at WPI-AIMR, remarked that this research sets a foundation for expedited materials screening and optimization. The overarching aim is to advance the design of more efficient and durable catalysts that can facilitate the widespread adoption of clean energy technologies, notably in hydrogen production.
As the global demand for sustainable energy solutions escalates, breakthroughs like these are essential in fostering innovations that not only enhance energy efficiency but also contribute to environmental sustainability. The journey toward cleaner energy continues, fueled by scientific ingenuity and commitment to excellence in research.