The quest for more efficient and safer energy storage options has led researchers to explore solid-state electrolytes, which serve as a vital component in the development of advanced battery technologies. Unlike traditional liquid electrolytes, which can pose safety risks due to leakage or flammability, solid-state electrolytes offer a promising alternative. These materials facilitate the efficient movement of ions within batteries, crucial for maximizing their energy capacity and overall performance. However, significant advancements are still needed to enhance the efficacy of current solid polymer electrolytes, thereby paving the way for next-generation energy storage solutions.
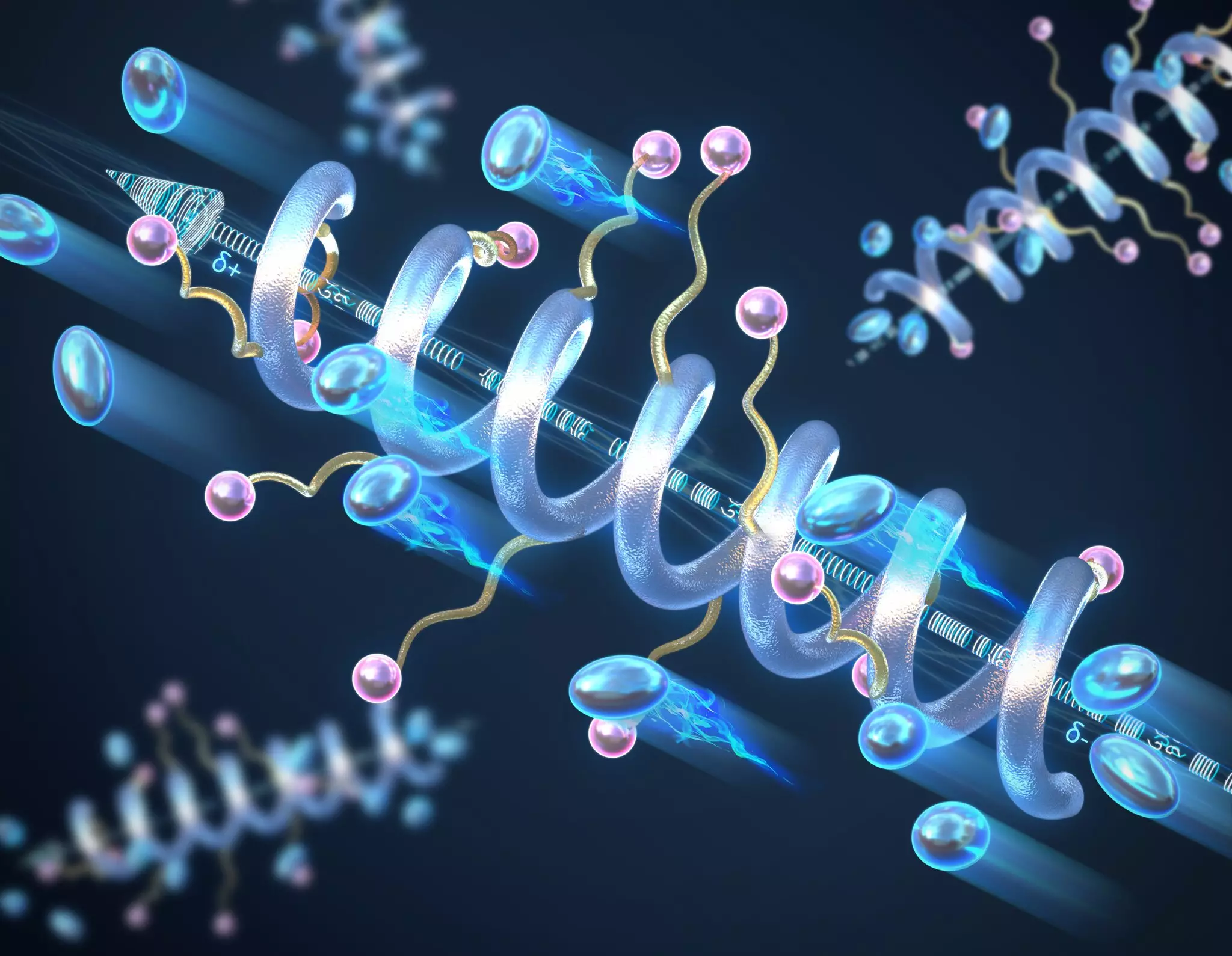
Research led by scientists at the University of Illinois Urbana-Champaign has illustrated the pivotal role of secondary structures, specifically helical configurations, in improving the conductivity of solid-state peptide polymer electrolytes. Utilizing a helical structure, similar to the double helix of DNA, enables these polymers to exhibit notably higher ionic conductivity when compared to their “random coil” counterparts. This revelation is particularly significant, as conductivity is a fundamental property that influences the performance of batteries. Through their work, the researchers have demonstrated that longer helical structures correlate with enhanced conductivity, stabilizing the materials against adverse conditions such as high temperatures and voltages.
What sets the helical structure apart from random configurations lies in its ability to create a macrodipole moment. The arrangement of charged units within the helix leads to an accumulation of small dipole moments, which, when combined, result in a substantial separation of positive and negative charges throughout the polymer. This phenomenon not only augments the conductivity of the material but also elevates its dielectric constant—a key factor in determining a material’s capacity to store electrical energy. As the helix lengthens, the overall conductivity increases, highlighting the potential of structural design in polymer chemistry to influence performance outcomes.
The findings from the University of Illinois group emphasize that helical polymers possess superior stability compared to conventional random coil polymers. This durability allows these materials to withstand high operational temperatures and voltages without succumbing to degradation. In traditional polymers, structural breakdown can compromise battery safety and efficiency, but the robust nature of the helical structure affords a new level of resilience. Researchers have noted no signs of breakdown in these helical polymers during testing, suggesting their potential for long-term application in energy storage systems.
Another compelling aspect of this research pertains to the environmental impact of the materials. The peptide-based nature of the polymers provides an avenue for easier degradation when the batteries reach the end of their life cycle. Utilizing enzymes or acids, the helical polymers can be broken down into individual monomer units. This capability allows for the recovery and possible reuse of starting materials, significantly reducing waste and promoting sustainability in energy storage solutions. As the world increasingly prioritizes environmental considerations in technology, such advancements could be crucial in moving toward more eco-friendly alternatives.
As the demand for efficient and sustainable energy storage technologies continues to rise, the evolution of solid-state electrolytes becomes paramount. The innovative use of helical structures to enhance the conductivity and stability of peptide polymer electrolytes presents exciting prospects in the landscape of battery technology. Groundbreaking research like that conducted by the University of Illinois not only pushes the boundaries of materials science but also contributes to the broader goal of creating a safer, more sustainable energy future. By combining engineering ingenuity with a focus on environmental responsibility, we move closer to realizing the full potential of solid-state batteries in next-generation applications.