In the face of escalating climate change and its associated repercussions, the urgency to find effective strategies for reducing carbon dioxide (CO2) emissions has never been more pressing. CO2 emissions are primarily associated with the production of electricity and heat, transportation, and various industrial processes. As researchers explore different methods to tackle this issue, electrochemical reduction emerges as a promising avenue. This method enables the conversion of recaptured CO2 into valuable products such as methanol and ethanol, potentially transforming a harmful pollutant into usable fuels. However, a significant barrier to the practical application of this process lies in the development of efficient catalysts that can operate at scale.
A recent innovative study led by scientists from the U.S. Department of Energy’s Brookhaven National Laboratory, in collaboration with Yale University and the University of North Carolina at Chapel Hill, has made substantial strides in overcoming the catalyst efficiency challenge. Findings published in the Journal of the American Chemical Society detail a promising approach that dramatically enhances the speed of catalysis—reportedly by a staggering factor of 800—without requiring substantial additional electrical energy input. This achievement is crucial considering the economic constraints presented by high energy demands typically associated with CO2 reduction.
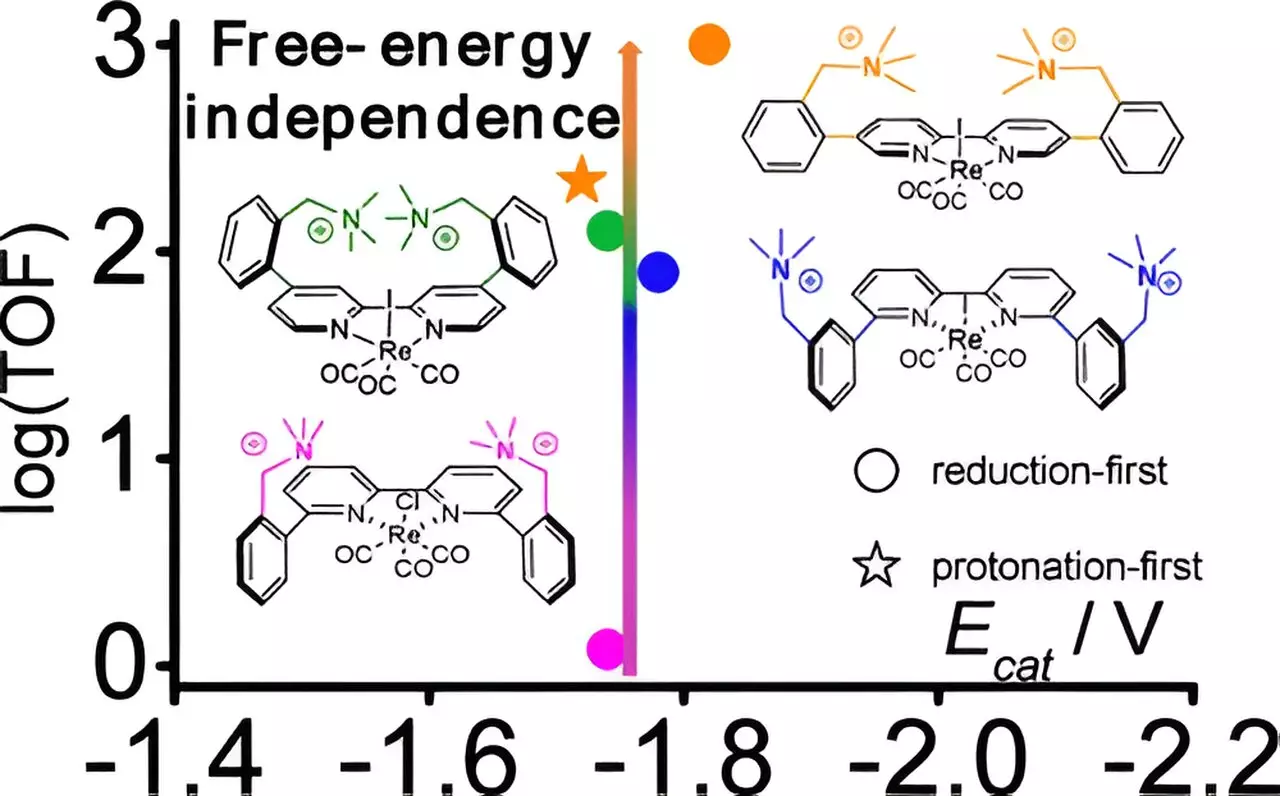
Central to the research is a catalyst grounded in rhenium, a metal that has shown potential in catalytic applications. The team of researchers discovered that by dexterously modifying the catalyst—more specifically, by decorating it with strategically placed positively charged molecules (cations)—they could influence the spatial arrangement between these cations and the rhenium core. Remarkably, adjusting this geometric spacing had a profound effect on the catalyst’s performance, unlocking new, lower-energy pathways for chemical reactions. Gerald Manbeck, a chemist at Brookhaven, emphasized that the evolution of this catalyst could spark the development of even more advanced catalytic systems in the future.
The researchers utilized advanced computational chemistry techniques to understand the underlying dynamics of their findings better. These methods revealed that the presence of cations stabilizes various stages of the catalytic reaction, enhancing overall efficiency. The computational models pointed towards a previously overlooked reaction pathway which became accessible uniquely due to the improved catalyst design. This innovative analytical approach synthesizes high-level science with practical applications, enabling more informed designs for next-generation catalysts aimed at facilitating CO2 reduction.
The study leveraged an array of experimental methodologies to validate its findings. Techniques such as cyclic voltammetry were employed to measure reaction rates, while infrared spectroelectrochemistry provided insights into the structural changes occurring during the catalytic process. A notable aspect of this research is the use of a novel apparatus that allows for heightened sensitivity in observing chemical transformations at the critical interface of the solution and electrode. This apparatus, developed by team members, represents a significant advancement, enhancing the group’s ability to monitor and understand the intricate processes involved during catalysis.
Looking forward, the research team is eager to build upon their discoveries by incorporating semiconductor-based light absorbers, such as silicon, into their catalytic systems. By harnessing solar energy, these light absorbers could provide a supplementary energy source, potentially lessening the dependence on direct electrical energy input. Such integration aligns seamlessly with broader initiatives like the CHASE program, focused on developing photoelectrodes that utilize sunlight to drive the conversion of CO2 and water into liquid fuels.
The strides made in this research provide a compelling glimpse into the future of carbon reduction technologies. As scientists build on these foundational discoveries, there is hope that new catalytic systems will emerge, capable of economically feasible and efficient CO2 conversion. By transforming CO2 into valuable resources while simultaneously addressing environmental concerns, these innovations could play a pivotal role in the transition to sustainable energy solutions. The work underscores a significant advancement in both our theoretical understanding and practical capabilities in the realm of catalysis and CO2 reduction, marking an important milestone towards a more sustainable future.