In the realm of battery technology, lithium and sodium are at the forefront of innovation, particularly in the development of solid-state batteries. High-performance electrochemical energy storage systems rely heavily on these alkali metals, which possess significant advantages over traditional lithium-ion systems. As the demand for more efficient and safe energy solutions continues to grow, the exploration of metal anodes has become essential. However, the intimate relationship between a metal’s microstructure and its electrochemical properties often goes overlooked—a gap that requires urgent attention if we are to tap into the full potential of lithium and sodium in battery applications.
In an unprecedented achievement, researchers from Justus Liebig University Giessen (JLU) and collaborating institutions in the U.S. and Canada have unveiled a novel method to elucidate the microstructure of these alkali metals when deposited in a battery environment. This groundbreaking work, published in the esteemed journal *Nature Materials*, sheds light on a subject that has been notoriously challenging due to the intrinsic reactivity of lithium and sodium.
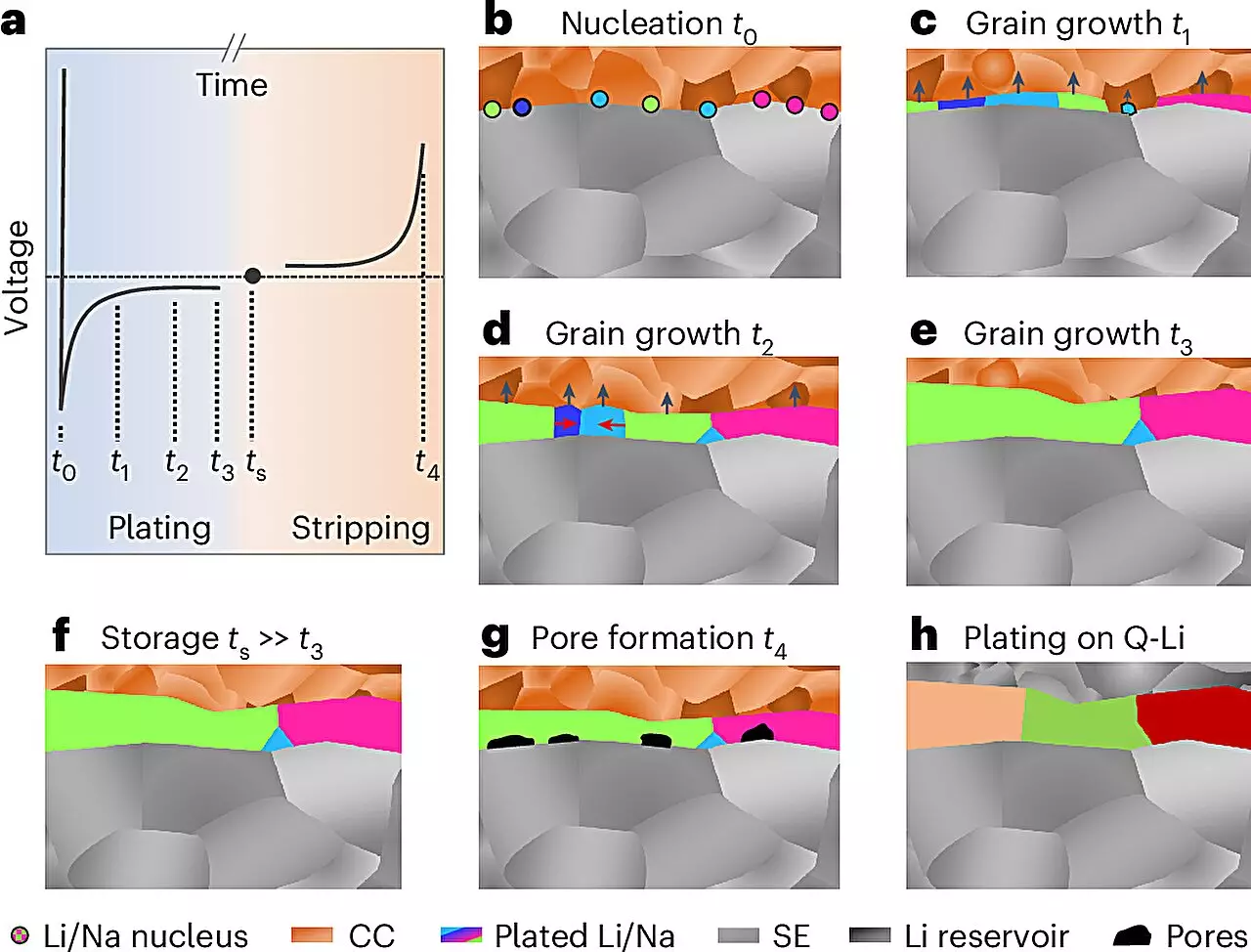
The properties of metals, particularly regarding their performance in electrochemical situations, depend significantly on their microstructure—the arrangement of crystallites and defects on nano- to micrometer scales. While this has been extensively explored in many commercial metals, lithium and sodium have remained largely underdetermined due to their quick reaction with environmental elements, which typically leads to an uneven and complex surface layer that complicates direct observation.
Methodology: Innovations in Analysis
The research team, led by Prof. Dr. Jürgen Janek from JLU, pioneered a series of meticulous preparatory and analytical steps performed under sharply controlled low-temperature and inert gas conditions. Through techniques such as electron backscatter diffraction, this method allows for a detailed examination of the structural nuances in lithium and sodium layers up to 100 micrometers thick. This novel approach not only provided insights into the grain sizes of the deposits but also revealed essential information regarding their growth mechanisms.
Prof. Janek remarked on the revelations these findings could usher in for the development of sodium batteries, a critical area of study within the broader POLiS (Post Lithium Energy Storage) excellence cluster. Understanding these microstructural characteristics could serve as the bedrock for building next-generation solid-state batteries capable of outperforming conventional lithium-ion technologies.
The transition towards solid-state batteries is fraught with challenges. Although ceramic solid electrolytes present a promising avenue for employing lithium and sodium metal anodes, the handling of these highly reactive metals brings forth significant operational hurdles. One prevalent issue is the deformation of metal during electrochemical cycling, which not only hampers charging and discharging performance but also leads to the formation of dendritic structures—unwanted tree-like formations that can provoke short circuits.
Ideally, for battery systems leveraging alkali metals, it is desirable that lithium or sodium only form during initial charging phases. This strategic timing could effectively mitigate the known complications associated with managing highly reactive alkali foil electrodes and curb the adverse effects of reactivity that current systems face.
This significant research advancement is a testament to the power of collaboration. The interplay of expertise from various domains of materials science, chemistry, and engineering enabled the successful identification and characterization of lithium and sodium microstructures. This cross-pollination of knowledge not only exemplifies the collaborative spirit of the research team at JLU but also underscores the global effort required in the push towards sustainable and powerful energy solutions.
As researchers continue to refine these findings and explore the implications for sodium batteries, the need for further investigation becomes evident. Understanding the nuances of these reactive metals’ behaviors opens the door to the optimization of battery designs that could potentially revolutionize energy storage technologies.
The groundbreaking work at JLU signals not just a pivotal leap in our understanding of lithium and sodium metal anodes, but also the immense potential for the future of solid-state batteries. By delving deeper into the microstructural dynamics of these alkali metals, researchers can pave the way for advanced energy storage systems that are not only powerful but also safe and enduring. The journey may be fraught with challenges, but with every discovery, we edge closer to realizing the full capabilities embedded in these fundamental components of modern electrochemical technology.