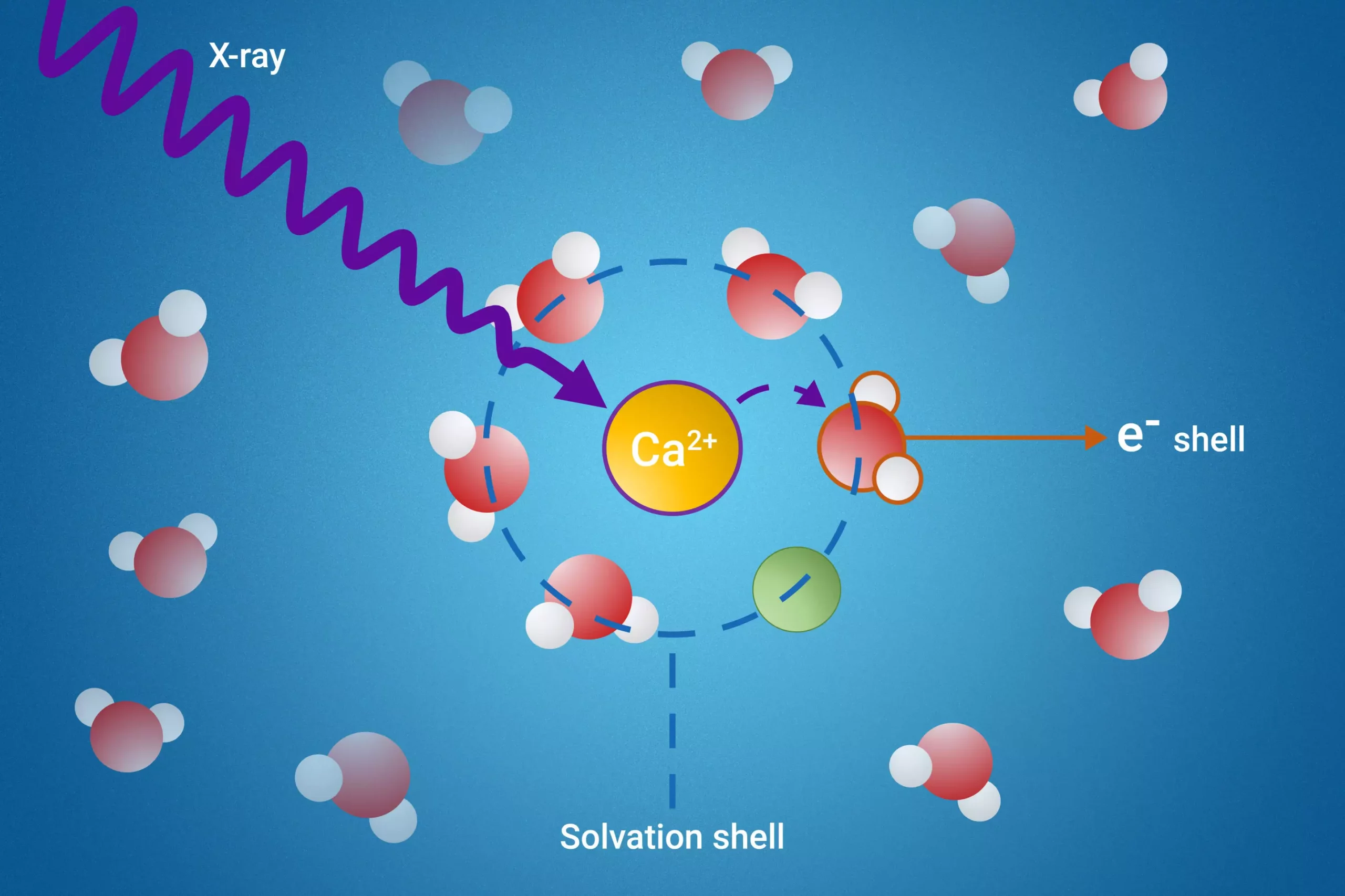
Recent advancements in our understanding of ion behavior in solutions have emerged from a collaborative effort involving researchers from the Fritz Haber Institute, Sorbonne University, and Uppsala University. Their published work in the esteemed journal Nature Communications presents a pioneering exploration into solvation shells—an essential phenomenon when examining how substances interact with solvents in liquid environments. When ions or particles dissolve in a solvent, they attract solvent molecules, forming a structured layer known as the solvation shell. This layer significantly alters the properties of the solvent, making it crucial to study. Nevertheless, the intricate nature of these shells and the difficulty in isolating their components have posed significant challenges for scientists.
The research team introduced a novel methodology employing resonant intermolecular Coulombic decay (ICD) to acquire insights into solvation shells. By utilizing X-ray excitation, they meticulously tracked the interactions between solvent molecules surrounding a dissolved particle as these molecules decayed. This technique allows scientists to observe how these solvation shells behave under specific conditions, opening up new avenues for investigation. Unlike traditional methods, which often provided limited information, this innovative approach has vastly improved the ability to analyze the properties associated with solvation shells, marking a substantial leap in the field.
Their research yielded critical insights into phenomena such as ion pair formation, a process where pairs of oppositely charged ions come together in solution. By studying the ICD process, the scientists identified a distinct pattern that serves as a reliable indicator of ion interactions within the solvation shell. Furthermore, the team successfully measured electron binding energies of the water molecules in the initial solvation shell—an achievement that has been notoriously difficult to quantify in prior research. This breakthrough is not just a technical triumph; it also enhances our comprehension of the dynamics of dissolution on a molecular level, providing a foundation for further scientific inquiry.
Understanding solvation shells is paramount for various scientific disciplines, ranging from chemistry and biology to materials science and electrochemistry. The implications of this research extend well beyond theoretical knowledge; they have profound consequences for practical applications, including drug formulation, battery technology, and environmental science. The capacity to explore solvation shells more comprehensively means that researchers can develop improved models for predicting solution behaviors, leading to advancements in innovative solutions for modern scientific challenges.
As scientists continue to probe the intricate world of molecular interactions, the refined techniques highlighted in this research offer a promising framework for future studies. Gaining clarity on the role and dynamics of solvation shells not only enriches fundamental scientific understanding but also equips researchers with the tools necessary to tackle pressing technological and environmental issues. Such breakthroughs lay the groundwork for future explorations into the nuanced behaviors of molecules in solutions, which could significantly transform several industries and scientific fields.