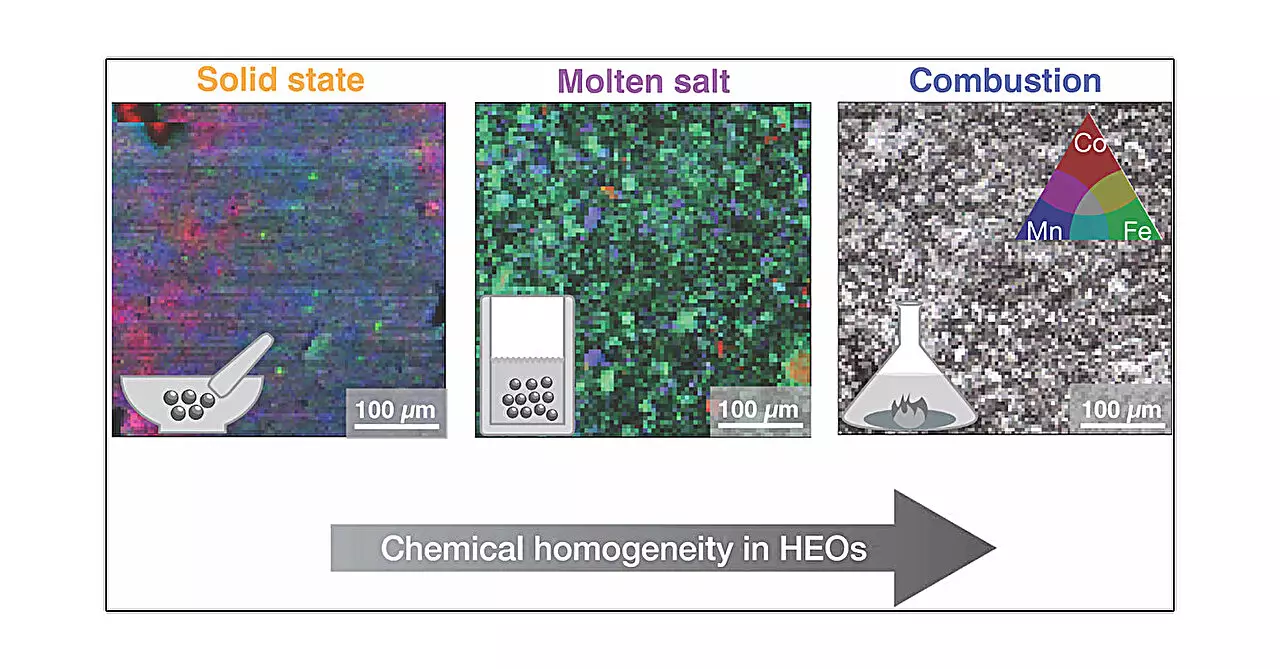
High entropy oxides (HEOs) are an exciting class of materials that have garnered attention for their remarkable properties, particularly in the context of electronic applications. Characterized by a structural complexity arising from the incorporation of multiple transition metal oxides, they present a unique opportunity for advancements in various technological fields. Recent research underscores the significance of synthesis techniques in determining the physical and electrochemical properties of these materials, showcasing how even subtle variations in preparation methods can have substantial implications for their functionality and effectiveness.
A pivotal study published in the Journal of the American Chemical Society reveals that the method used to synthesize HEOs is critical in defining their performance characteristics. Every synthesis technique—from solid-state synthesis to advanced combustion methods—introduces distinct influences on the material’s microstructural arrangement. This emphasizes the notion that two ostensibly identical HEO samples can exhibit vastly different behaviors depending on their synthesis routes. The researchers employed five distinct techniques: solid-state, high pressure, hydrothermal, molten salt, and combustion synthesis, all of which vary in their thermal profiles and chemical environments during production.
Insights from the Research
This research, led by Alannah Hallas and her team at the University of British Columbia, provides compelling evidence supporting the argument that synthesis methods profoundly affect not just the microstructure but also the local arrangements of atoms within the material. Hallas articulates that the excitement surrounding HEOs stems from their inherent chemical flexibility, which opens up a myriad of potential applications across different sectors. For instance, the study demonstrated that while the average atomic structure remains consistent across different methods, the local structural characteristics and resultant properties can diverge significantly.
The lead author, Mario Ulises González-Rivas, elaborates on the nuances of each synthesis approach, indicating that the driving forces behind the formation of HEOs are inherently tied to the chosen method. For example, the solid-state method can be likened to conventional baking, where the right mix of components must meet specific heat conditions. On the other hand, high-pressure synthesis introduces additional external forces, potentially enhancing the crystallization process. Understanding these mechanisms reveals how precise control over synthesis can tailor material properties for specific applications, making them even more appealing for practical use in energy systems and beyond.
As interest in energy-efficient technologies continues to grow, the adaptability of HEOs positions them as viable contenders for addressing urgent energy challenges. From batteries to fuel cells, the electrochemical properties of HEOs can significantly impact the efficiency and longevity of energy systems. The study’s findings lay the groundwork for future optimization strategies, suggesting that material engineers and researchers may now have a new axis to investigate when devising technology using these materials.
The interplay between synthesis methods and resultant material properties also opens the door for innovative research directions. By exploring new synthesis techniques or fine-tuning existing ones, scientists may create HEOs with enhanced performance metrics tailored to meet industry-specific needs. The collaborative efforts among institutions, as represented by prominent contributors from the University of Saskatchewan and the Max Planck Institute for Solid State Research, highlight the interdisciplinary nature of materials science and the importance of global collaboration in pushing the boundaries of material functionality.
The study on high entropy oxides illuminates the pivotal role that synthesis methods play in shaping material properties and potential applications. By gaining insights into how different methods affect structural and functional characteristics, researchers can better harness the benefits of HEOs for technological advancement. This research not only propels our understanding forward but also sets the stage for future innovations in materials science, where optimization of synthesis will be central to developing next-generation electronic and energy systems.