Polymers are fundamental components of modern life, utilized in everything from everyday products to complex pharmaceuticals. Traditional polymer chemistry has primarily relied on well-established methodologies, which, while effective, often limit the types of materials that can be synthesized and their subsequent applications. Scripps Research chemists, in collaboration with prominent institutions such as the Georgia Institute of Technology and the University of Pittsburgh, have taken a significant step forward by developing a novel reaction using nickel as a catalytic element. Their groundbreaking work promises to open new pathways for creating polymers with tailored properties, enhancing various industrial sectors.
To conceptualize polymers, one can liken them to a train made up of numerous cars. Each car, or monomer, is linked by couplings that represent the chemical bonds between them. The diversity of a train’s journey is paralleled by the functionality of polymers; both are shaped by the nature of their building blocks. Traditionally, the challenge faced by chemists has been that these building blocks, or monomers, were chemically similar, thus constraining the variety of resulting polymers to limited applications. The emergent nickel-catalyzed method changes this, permitting the crafting of unique monomers in a controlled manner.
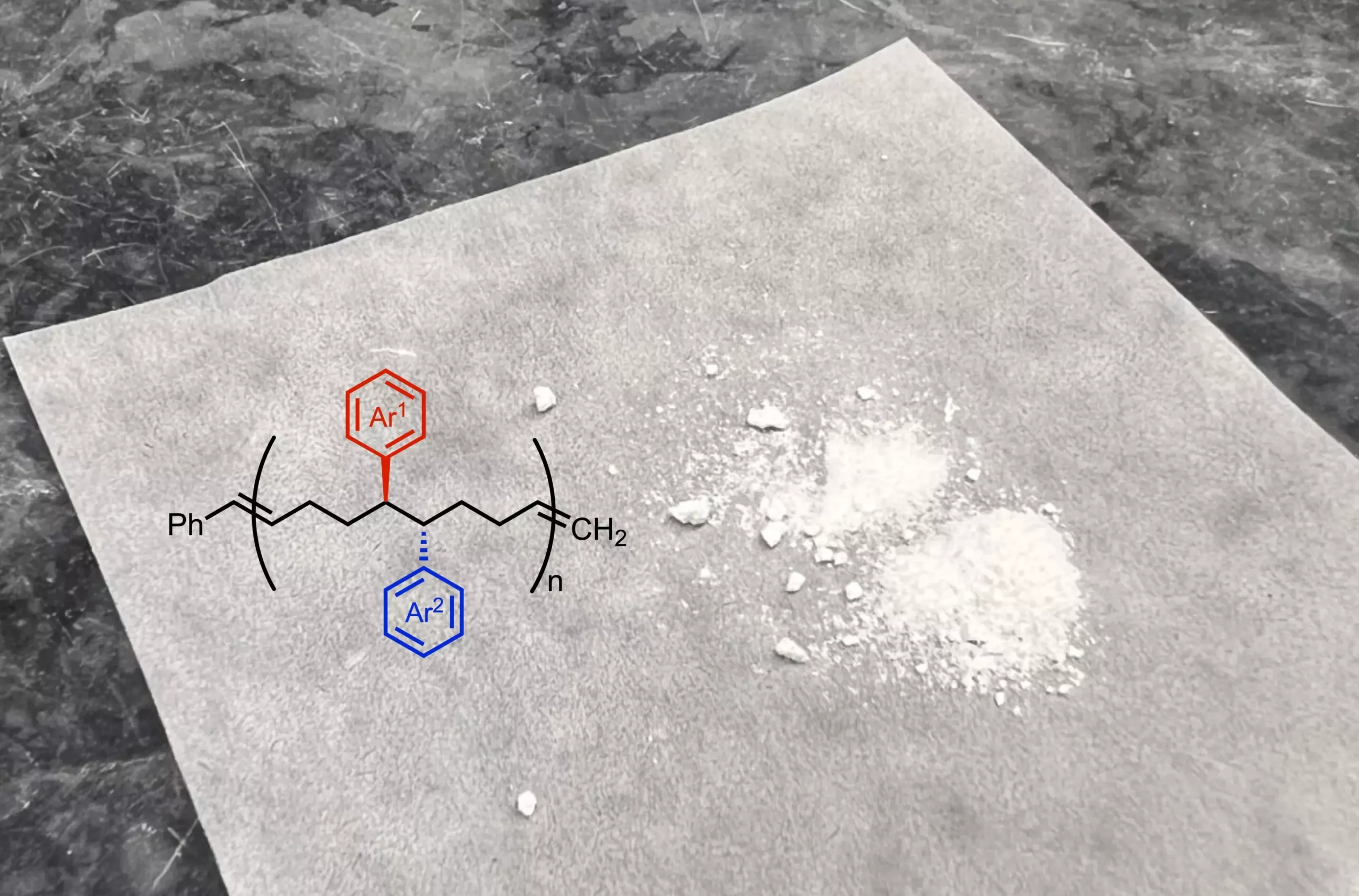
A pivotal aspect of this new reaction is its capability to introduce two distinct functional groups to the monomers. These groups are small side chains that impart unique chemical and physical characteristics to the resulting polymers, impacting factors such as flexibility, elasticity, and solubility. The senior author of the study, Dr. Keary Engle, emphasizes that this advancement not only broadens the structural diversity of polymers but also enhances their applicability across numerous domains, including drug delivery systems, energy storage solutions, and microelectronics.
Anne Ravn, a postdoctoral researcher and co-first author of the study, discusses the crucial nature of modifying the building blocks. As she points out, the arrangement of these functional groups directly influences the final properties of the polymer, enabling researchers to tailor materials to specific needs.
This project stands as a testament to the power of collaboration in scientific inquiry. The diverse backgrounds of the researchers involved have enriched the study, with contributions spanning from small molecule development to advanced polymer research. Co-first authors include graduate students and researchers from various institutions, bringing a wealth of knowledge and expertise to the table. Such collaboration highlights the interdisciplinary nature of modern scientific challenges and solutions, allowing for greater innovation.
The initial success of producing unique monomers has led to further investigations into scaling up these methods for industrial applications. The team at Georgia Institute of Technology has been instrumental in polymerizing these new monomers, demonstrating that the benefits of the nickel-catalyzed reaction can indeed be realized on a larger scale. The researchers are keen to exploit this methodology for various real-world applications, thereby significantly expanding the utility of synthetic polymers.
The conventional polymer structure, characterized by uniformly spaced carbon links between functional groups, has been transformed by this technique. In the new polymers synthesized through nickel catalysis, functional groups are placed closer together. This spatial arrangement results in an entirely different set of material properties, potentially revolutionizing how industries can engage with polymers.
One of the compelling advantages of utilizing nickel as a catalyst is its abundance compared to other metals typically employed in similar reactions. This shift not only suggests a more resource-efficient approach to polymer synthesis but aligns with growing environmental concerns regarding sustainable production methods. The researchers are dedicated to exploring eco-friendly ways to further enhance the sustainability of their polymer products.
A significant focus moving forward will be on developing methodologies for the degradation of these new polymers back into their original building blocks. This initiative aims to create a circular economy within polymer production, where materials can be reused and recycled, minimizing waste and environmental impact.
The innovative research led by Scripps Research, alongside its collaborators, has ushered in a new era of polymer chemistry marked by the use of nickel catalysis. With the potential to create unique monomers and, consequently, diverse polymers with tailored functionalities, the implications for various industries are vast. As researchers continue to explore and refine these methods, the promise of sustainable, versatile polymer synthesis is not just theoretical but increasingly within reach, heralding exciting developments for the future of materials science.