Alzheimer’s disease, a complex and multifaceted neurodegenerative disorder, has long been associated with amyloid plaques—abnormal clumps of protein found in the brains of those affected. For years, research efforts have concentrated on diminishing the presence of these amyloid fibrils, with the prevailing belief that their accumulation directly contributes to the onset and progression of dementia. However, recent insights into quantum biology may provide a new perspective on both the role of amyloid in the context of Alzheimer’s and the prospects for effective treatment strategies.
The dual struggle against amyloid accumulation and oxidative stress has led to treatment approaches focused predominantly on these entities. Yet an intriguing discrepancy persists; many individuals harbor significant levels of amyloid without exhibiting cognitive decline, challenging the notion that amyloid is inevitably pathological. The complexity of Alzheimer’s requires an examination of other contributing factors, such as allostatic load—a term denoting the cumulative physiological burden imposed by stressors over time. Biochemical markers signaling oxidative stress, often resulting from the excessive generation of free radicals, are increasingly recognized as significant players in the disease’s pathology.
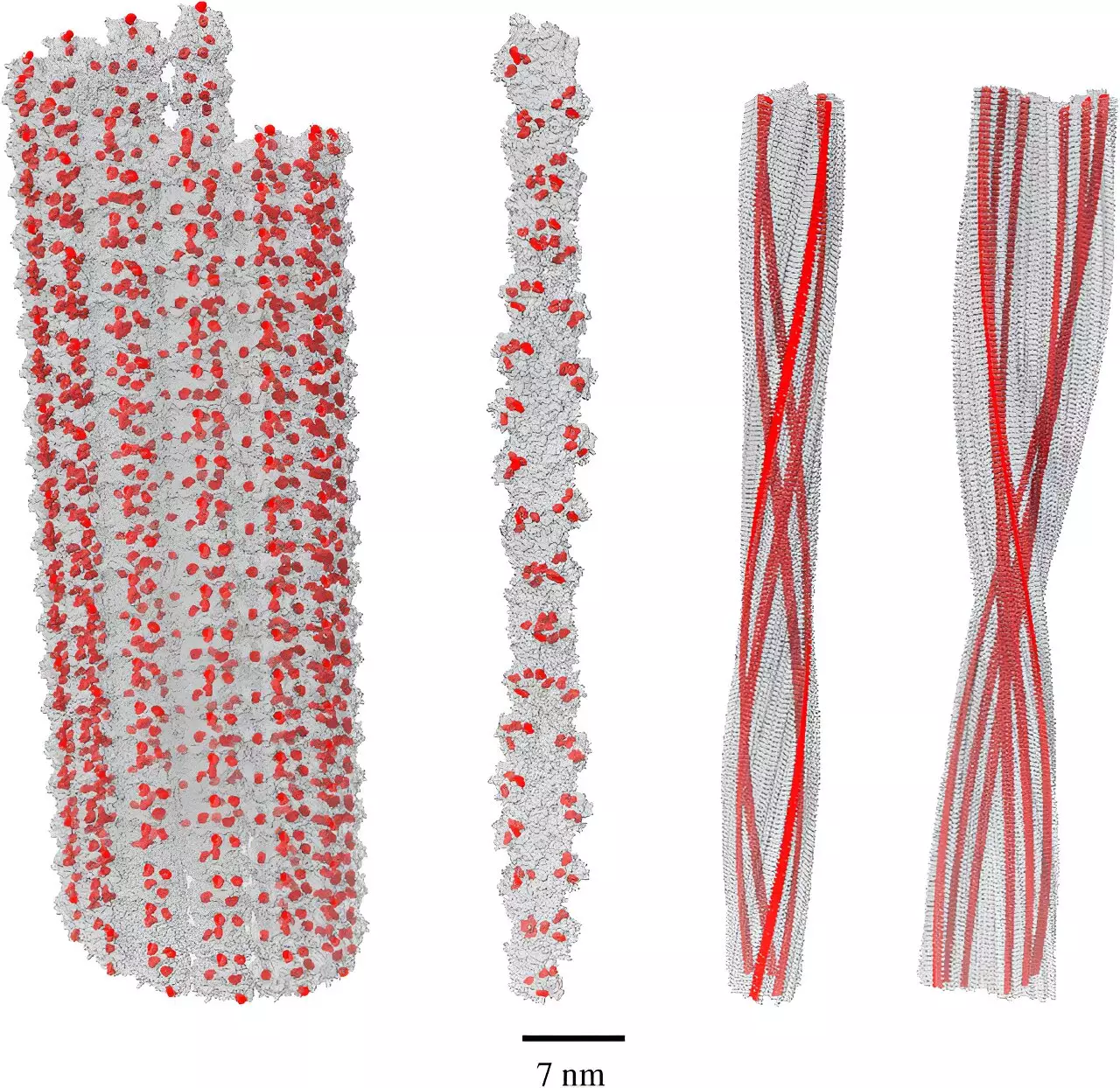
Recent findings by Dr. Philip Kurian and his team at the Quantum Biology Laboratory at Howard University have revealed remarkable properties of amino acid networks, particularly those involving tryptophan, a precursor for serotonin. Their exploration of a phenomenon known as single-photon superradiance may hold the key to rerouting our understanding of amyloid fibrils’ role in Alzheimer’s.
In simpler terms, single-photon superradiance refers to how a collective group of molecules can efficiently absorb harmful high-energy photons—often emitted during oxidative stress—and re-emit this energy at a considerably lower, non-damaging level. This energy down-conversion process not only serves as a protective mechanism but also raises questions about whether amyloid, instead of being a culprit, could serve as an adaptive response to environmental stressors.
Dr. Kurian’s research indicates that amyloid fibrils exhibit an enhanced capacity for superradiance, potentially up to five times greater than individual tryptophan molecules. This revelation suggests a paradigm shift: rather than contributing to cognitive decline, amyloid aggregation may instead be an evolutionary strategy to cope with oxidative stress and protect neuronal integrity.
The implications of these findings are profound. For decades, pharmaceutical interventions focused on the reduction of amyloid levels as a primary target in Alzheimer’s treatment have yielded limited success. Kurian’s discoveries challenge this approach, suggesting the need to investigate alternative strategies that enhance the protective functions of amyloid fibrils rather than eliminate them outright.
Professor Lon Schneider, a prominent figure in Alzheimer’s research, lauded these contributions as critical to reshaping our understanding of the disease’s pathology. The inference that amyloid formations could signify a protective mechanism rather than a pathogenic one might necessitate a re-evaluation of current clinical practices and research funding priorities. If amyloid fibrils indeed serve a beneficial role amid neurodegeneration, it could signal a substantial pivot in the approach to therapeutic development.
Dr. Kurian’s vision extends beyond the immediate implications for Alzheimer’s research; he advocates for a broader integration of quantum perspectives in life sciences. As the study of quantum biology continues to evolve, it becomes essential to consider how quantum mechanics informs interactions at the molecular level in living systems.
This multidisciplinary approach, blending open quantum systems with computational biology and photophysics, emphasizes the interconnectedness of biological phenomena. Mr. Hamza Patwa, a contributing scholar to the research, underscores the importance of breaking down silos within scientific disciplines to yield innovative insights into complex problems.
As the scientific community prepares to validate these intriguing predictions experimentally, the quest to understand Alzheimer’s more holistically is underway. The recognition that quantum effects and biochemical networks contribute meaningfully to neurobiology indicates a promising frontier for future research.
As we continue exploring these layers of complexity within protein structures, we might redefine the landscapes of treatment and prevention strategies. The synthesis of quantum mechanics and biology not only paves the way for paradigm shifts in our understanding of neurodegenerative diseases but holds the potential to unlock transformative therapeutic avenues. Engaging with and embracing these multifaceted interactions may be the key to advancing Alzheimer’s research and, ultimately, improving outcomes for those affected by this devastating disease.