Ammonia plays a pivotal role in global agriculture as a primary ingredient in fertilizers, and it has emerged as a potential zero-carbon fuel source owing to its high energy density and clean combustion. However, the traditional method of synthesizing ammonia—known as the Haber-Bosch process—comes with significant drawbacks, notably its staggering energy consumption and corresponding contribution to climate change, accounting for approximately 1.8% of global carbon dioxide emissions. Given the urgent need to mitigate these environmental impacts while fulfilling ammonia’s critical role in food production and energy, researchers are exploring innovative pathways to synthesize ammonia that are more energy-efficient and environmentally friendly.
Recent research published in ACS Nano presents a compelling advancement in the field of electrochemical nitrate reduction reactions (eNO3RR), which converts nitrate directly into ammonia. This novel approach not only addresses the pressing need for energy-efficient ammonia production but also simultaneously tackles nitrate pollution, thereby acting as a dual solution for agricultural and environmental concerns. The study revealed that catalysts made from spinel cobalt oxide (Co3O4) could significantly enhance the efficiency of this process.
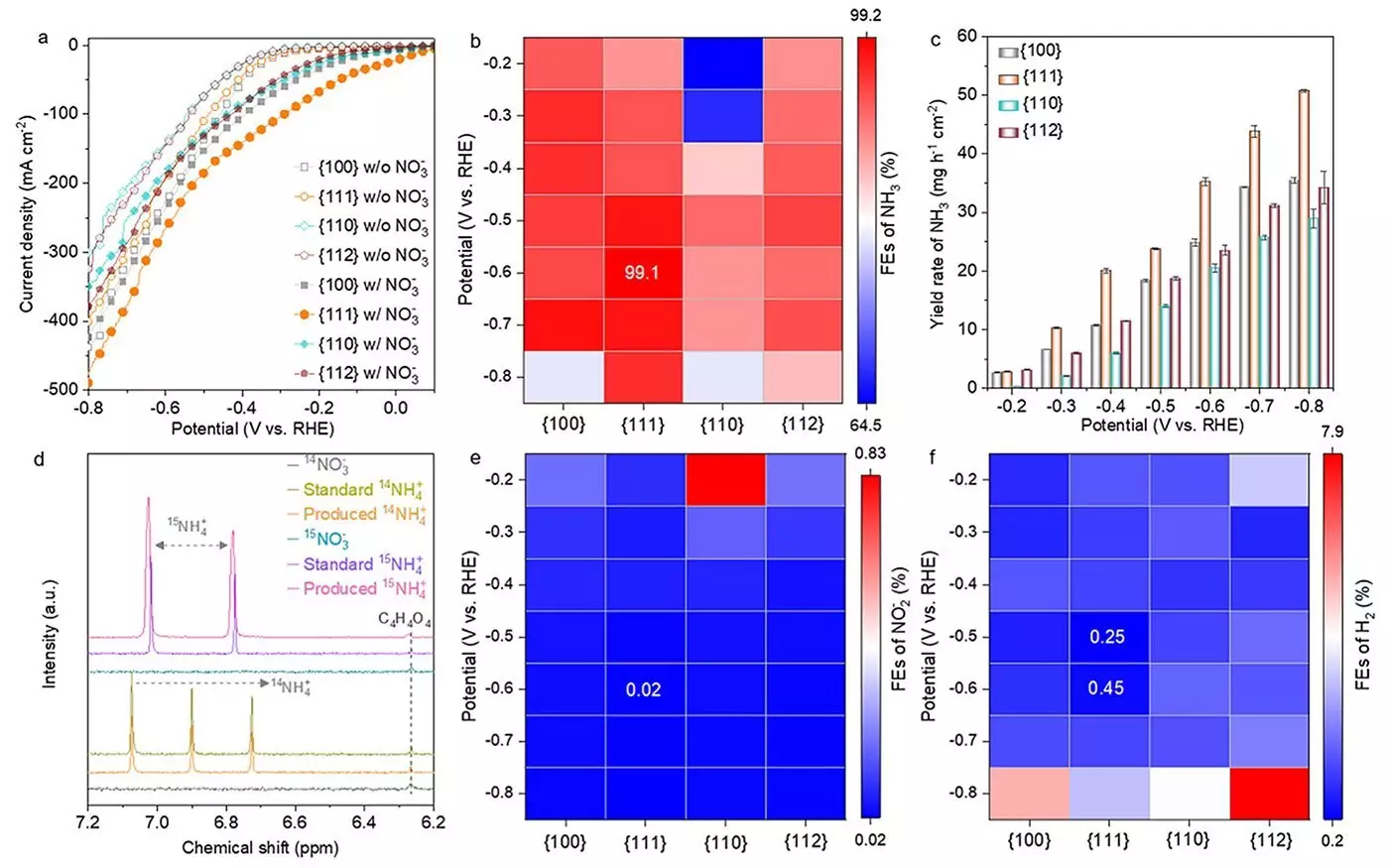
The research team focused on synthesizing different nanostructures of Co3O4, each exhibiting distinct crystallographic facets—{100}, {111}, {110}, and {112}—to determine their respective effects on catalytic performance. The initial findings pointed towards the {111} facet as the standout performer, achieving an astronomical ammonia Faradaic efficiency of 99.1%, coupled with a yield rate of 35.2 mg h-1 cm-2.
Dr. Heng Liu, a co-first author of the study, attributed the enhanced efficacy of the {111} facet to the swift generation of oxygen vacancies, and the formation of Co(OH)2, which seems to significantly optimize the conversion process. This illuminates an essential aspect of catalyst performance not merely tied to the composition and structure but also to the transient phases that catalysts undergo during reaction processes.
As the reaction proceeds, it is fascinating to note that the catalyst transitions through various structures—from Co3O4 to an oxygen-deficient state, and then finally forming a hybrid structure of Co3O4−x−Ov/Co(OH)2. Understanding these transformations is crucial as they hold the key to unraveling the mechanics behind catalyst activation and longevity. Professor Hao Li highlighted that grasping these intricate changes can significantly inform future catalyst designs aimed at achieving even higher levels of activity and selectivity.
The implications of this breakthrough extend beyond the laboratory, promising transformation across various industrial applications. Not only does eNO3RR present an alternative method of ammonia production with a considerably reduced carbon footprint, but it also doubles as a remedy for nitrate waste from agricultural runoff—thereby enhancing the overall sustainability of farming practices. In a world increasingly directed towards decarbonization, ammonia synthesized through this novel pathway could serve as a crucial player in the development of renewable energy systems.
Furthermore, as industries shift towards greener practices, optimizing the design of catalysts such as Co3O4 holds the immense potential to facilitate cleaner practices in chemical manufacturing, showcasing a significant leap toward carbon neutrality by the mid-21st century. “Our findings provide a strong foundation for engineering superior catalysts,” stated Li, pointing towards a future where sustainable industrial processes are not just aspirational but tangible.
In review, the research into Co3O4 catalysts for electrochemical nitrate reduction heralds a pivotal moment in ammonia production and environmental sustainability. As the global community grapples with climate challenges, the journey towards efficient and eco-friendly industrial practices becomes ever more critical. By harnessing innovative catalytic processes, we may transform some of today’s most pressing environmental issues into opportunities—turning nitrate pollution into a valuable resource while slashing the carbon emissions associated with ammonia synthesis. This work does not only enrich the scientific discourse but also propels us toward a more sustainable and resilient future.