In an era where antibiotic resistance poses a daunting challenge to modern medicine, a groundbreaking antibiotic class known as macrolones may herald a new age in the fight against infectious diseases. Research from the University of Illinois Chicago uncovers a dual-action mechanism that could make it exceedingly difficult—by a staggering factor of 100 million—for bacteria to evolve resistance. This pivotal development is not just a technical advancement; it represents a paradigm shift in our understanding of how to combat bacterial infections effectively.
The Science Behind Macrolones
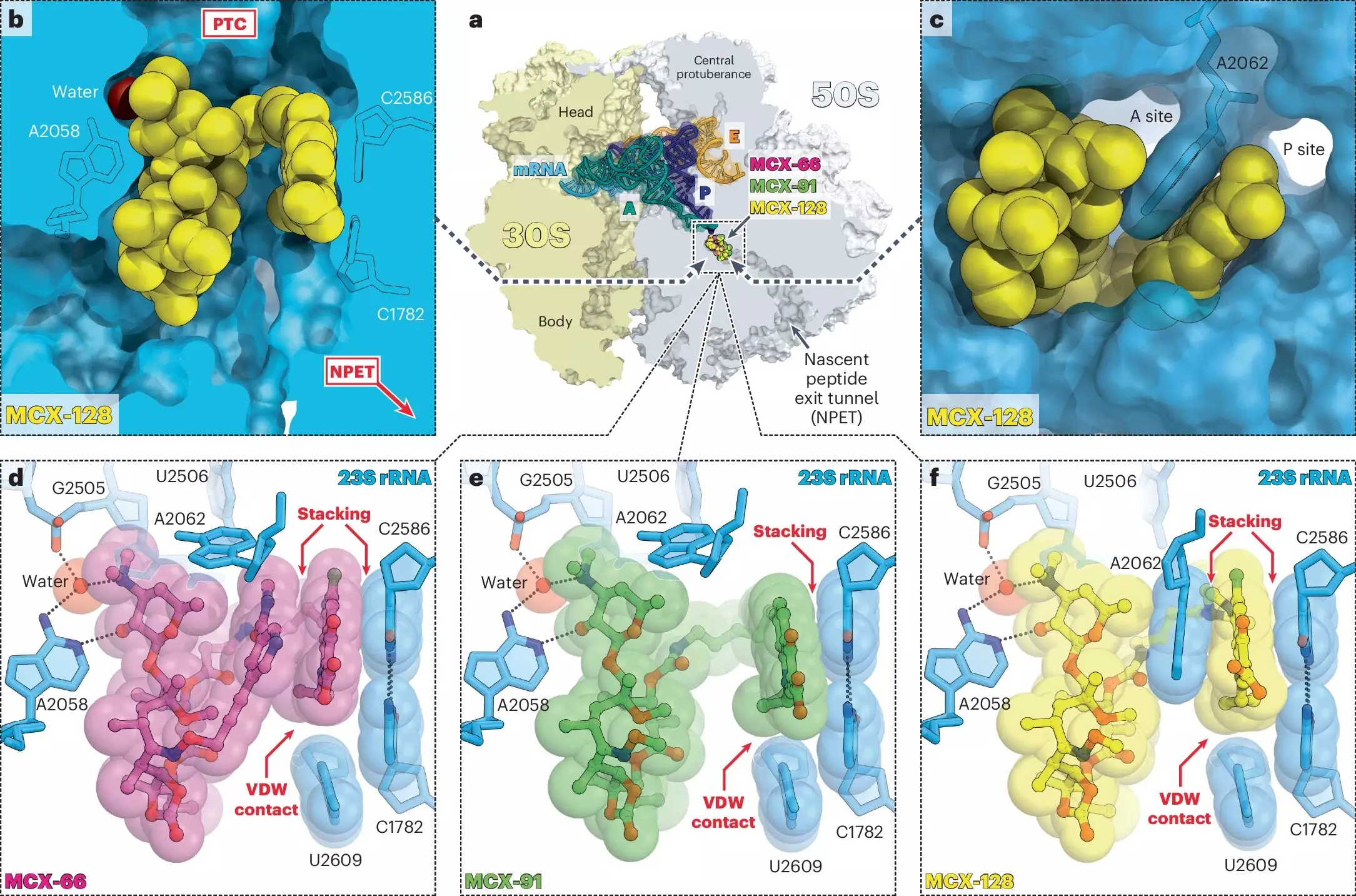
The strength of macrolones lies in their unique ability to disrupt bacterial cell function on two fronts. Unlike traditional antibiotics that typically target a single biological process, macrolones simultaneously interfere with protein synthesis and DNA integrity. By examining how these synthetic drugs function, researchers have revealed a multi-layered attack strategy that enhances efficacy and limits the likelihood of resistance.
Dr. Alexander Mankin, a distinguished professor of pharmaceutical sciences, succinctly articulates this advantage: “If the antibiotic hits both targets at the same concentration, the bacteria lose their ability to become resistant via acquisition of random mutations in any of the two targets.” This profound insight not only challenges the existing paradigm of antibiotic design but also underscores the critical need for innovative approaches to address the growing threat of resistant bacterial strains.
Mechanisms of Action: Targeting Bacteria Effectively
The structure of macrolones is remarkable, combining elements from two well-established antibiotic classes—macrolides and fluoroquinolones. While macrolides like erythromycin obstruct the ribosome, halting protein production, fluoroquinolones such as ciprofloxacin focus their assault on bacterial DNA gyrase, a pivotal enzyme in DNA replication.
The research spearheaded by Yury Polikanov and his colleagues has revealed that macrolones can bind with even greater efficacy than traditional macrolides. These drugs can inhibit the function of ribosomes in previously resistant bacterial strains while cleverly circumventing the activation of resistance genes. This duality not only expands the arsenal of drug options available to healthcare providers but also enhances treatment outcomes for patients suffering from stubborn infections.
Maximizing Potential Through Interdisciplinary Collaboration
One of the most striking aspects of this research is the collaborative environment at the UIC Molecular Biology Research Building. It exemplifies how interdisciplinary teamwork can spur significant scientific advancements. By bringing together experts from different fields, the researchers are able to share insights and data that may have been overlooked in a more siloed approach.
The study serves as a reminder that true innovation often occurs at the intersection of diverse academic disciplines. Through this collaborative lens, researchers can continue to optimize macrolones for maximum efficacy against bacterial pathogens. This dialogue between chemists and biologists is crucial in refining drug designs that effectively target both the ribosome and DNA gyrase.
The Implications for Future Research and Healthcare
The discovery of macrolones not only has immediate implications for the future of antibiotic development but also sets the stage for a renewed focus on understanding antibacterial mechanisms in greater depth. As bacteria become increasingly adept at evading conventional treatments, the need for novel solutions has never been more pressing.
The insights garnered from this research may well inform future efforts to create next-generation antibiotics that are more resilient against resistance mechanisms. Indeed, the ability of macrolones to deliver simultaneous strikes against multiple biological targets opens up avenues for developing a new class of antibiotics that could fundamentally change how we treat infections.
The University of Illinois Chicago’s exploration into macrolones is a testament to the power of innovative thinking in addressing one of humanity’s most urgent health crises. Not only does it unveil a promising new line of defense against bacterial resistance, but it also emphasizes the essential role of interdisciplinary collaboration in pushing the boundaries of scientific discovery. Such efforts are crucial in shaping the future landscape of antibacterial therapies, and they inspire hope in an otherwise challenging health environment.