The global drive towards sustainable industrial practices has intensified research efforts focused on the conversion of biomass into essential chemical precursors, such as olefins. These precursors serve as building blocks for various products, from plastics to pharmaceuticals. A recent study from Kyushu University has highlighted a novel approach using a zeolite material known as Na-ZSM-5, employing microwave heating to streamline this conversion process. This breakthrough has the potential to significantly impact the energy consumption and environmental footprint of the chemical industry.
The conventional synthesis of chemicals often relies heavily on processes like naphtha reforming, which requires significant energy inputs and generates substantial carbon dioxide emissions. Given the growing concerns about climate change and the urgent need for greener methodologies, researchers have turned their attention to alternative biomass sources, such as cooking oil waste and microalgal oils. These sources present a feasible route for generating the simpler chemicals required for complex organic synthesis through techniques like catalytic cracking, utilizing natural zeolites as catalysts.
However, the catalysis process traditionally necessitates temperatures ranging from 500 to 600 degrees Celsius, resulting in high energy consumption and coking—a detrimental build-up of deposits that compromises catalyst efficacy. Consequently, the quest for more energy-efficient methods is paramount in advancing sustainable chemical manufacturing.
The innovative work by Associate Professor Shuntaro Tsubaki and his research team sheds light on how microwave heating can address the challenges inherent in catalytic biomass conversion. By directly interacting with materials, microwaves facilitate selective heating, minimizing energy wastage as compared to conventional heating techniques. This method also promotes gas-solid catalysis by generating localized ‘hot spots’ in the solid catalyst without adversely affecting its structural integrity.
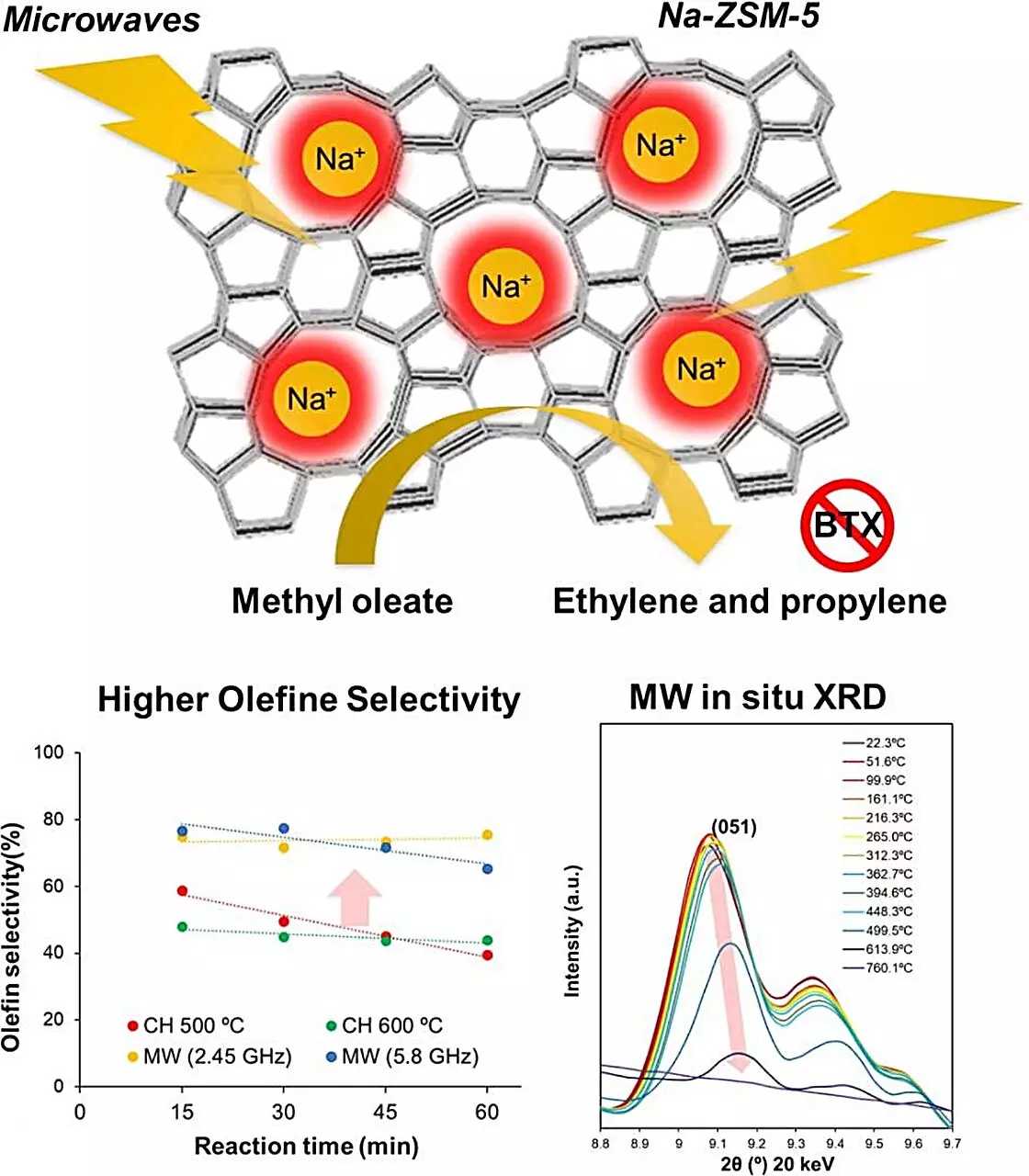
In their research, Tsubaki and his team concentrated on evaluating various zeolite catalysts, ultimately identifying Na-ZSM-5 as a standout option. Key to their findings was the observation that microwave heating offered substantial advantages over traditional heating methods, particularly in terms of conversion efficiencies and byproduct management. The experimental results demonstrated that microwave-activated Na-ZSM-5 achieved impressive conversion rates for fatty acid esters, substantially reducing carbon dioxide emissions and eliminating carbon monoxide production entirely.
One of the most remarkable outcomes of the study was the sharp increase in olefin production when Na-ZSM-5 was subjected to microwave heating, surpassing traditional heating methods by fourfold. This improvement correlates with the zeolite’s selective catalytic properties, favoring olefin formation over other chemical pathways. Significantly, the study found no occurrence of coking even at higher temperatures of 600 degrees Celsius, confirming that microwave heating not only elevates production output but also enhances catalyst longevity.
Furthermore, through detailed analysis, the researchers discovered that localized temperatures exceeding 1000 degrees Celsius were achieved within the crystal lattice of the zeolite during microwave exposure. This phenomenon is believed to be a crucial factor in the selective formation of desired olefins, demonstrating the profound impact of microwaves on the catalytic process.
The implications of these findings extend far beyond academic interest; they present a roadmap for the future of sustainable chemical production. Tsubaki emphasizes that this microwave-driven approach holds the potential for integrating renewable energy sources, such as solar and wind power, into chemical synthesis. By leveraging these technologies, the industry can significantly lessen its environmental impact while still meeting global chemical demands.
The team’s commitment to enhancing microwave-driven catalytic processes reflects a broader ambition to scale up these methodologies for real-world applications. Continuous improvements in yield and energy efficiency are essential to realizing these goals. As the chemical industry grapples with the dual challenges of maintaining productivity and addressing sustainability concerns, microwave-assisted catalysis might emerge as a transformative solution.
The work conducted by the researchers at Kyushu University is a compelling illustration of how innovative technologies can overcome longstanding barriers in biomass conversion. By harnessing the power of microwave heating in conjunction with zeolite catalysts, they not only present a more efficient method of producing crucial chemical precursors but also pave the way for a greener future in chemical manufacturing. The forthcoming enhancements in this technology could soon gift industries with the tools needed to transition into an era defined by sustainability and reduced environmental footprints. As the world pivots towards more responsible production, such revolutionary advancements will be critical in shaping a cleaner, more sustainable chemical landscape.