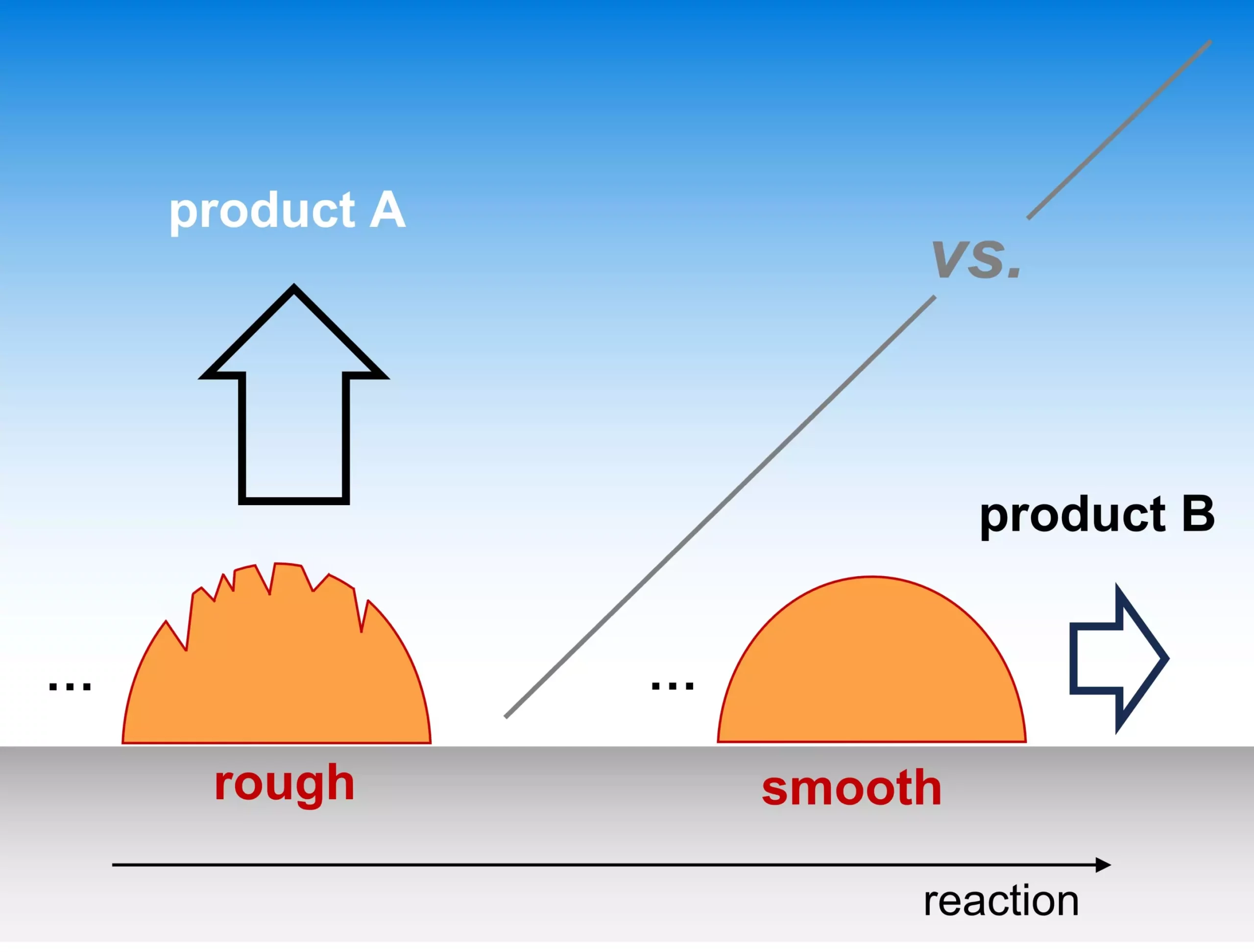
Recent revelations from the Theory Department of the Fritz Haber Institute highlight an intriguing aspect of catalysis that has been overlooked for far too long: the morphology of catalysts. Traditionally, the industry has concentrated on the atomic-level characteristics of active sites when assessing selectivity in electrocatalytic reactions. However, a groundbreaking study published in Nature Catalysis posits that the “roughness” of a catalyst’s surface significantly influences which products emerge from electrochemical processes, particularly in pivotal reactions such as converting CO2 into usable fuels or generating H2O in fuel cells. This insight not only broadens our understanding of catalysis but also opens new avenues for engineering efficient and sustainable chemical reactions.
The Role of Roughness in Selectivity
The team’s findings advocate for a paradigm where catalyst surface roughness is treated as a critical parameter, rather than an incidental characteristic. When electrocatalytic reactions occur, the microscopic mechanism often involves reaction intermediates escaping from the catalyst surface and appearing as products. While the depth of this process has historically been shrouded in mystery, the researchers provide substantial evidence that the rate of transport of these species within the electrolyte is intrinsically linked to the catalyst’s surface morphology. The roughness facilitates or hinders the movement of reaction intermediates, thereby influencing the selectivity for desired products.
Transforming Sustainability in Chemical Processes
Catalysis is not merely an esoteric field; it is foundational to various sectors that underpin modern civilization, including pharmaceuticals, fertilizers, and plastics. With the pressing demands of climate change, heterogeneous electrocatalysis stands out as a pivotal technology in transitioning towards carbon-neutral energy solutions. This method emphasizes producing essential fuels and chemicals by harnessing renewable electricity while operating under benign conditions. By emphasizing the influence of catalyst morphology, we now have a better grasp of how to refine these catalytic processes and subsequently contribute to a more sustainable future.
Implications for Future Research and Industry Practices
The simplistic yet effective multi-scale kinetic model introduced by the Fritz Haber research team underscores the practicality of their findings. This model quantifies how various factors, including the density of active sites—essentially, the surface roughness—affect selectivity. By successfully replicating several experimental findings using this model, the researchers demonstrate the broader applicability of their approach. As industries strive for efficiency in energy conversion and chemical production, a fundamental understanding of how to manipulate surface morphology is invaluable for enhancing catalyst performance.
A Call to Action for Innovators
The implications of these findings are profound not just for theoretical research but also for practical applications. As we face an energy crisis exacerbated by pollution and climate change, innovation in catalyst design must incorporate morphology as a vital consideration. Engineers and scientists must collaborate to rethink the methodologies of catalyst production, shifting focus from merely optimizing active sites to contemplating the intricate details of surface texture. As these discoveries gain traction, they are expected to catalyze a new wave of advancements in sustainable energy technologies, ushering in an era where catalysis is a cornerstone in achieving environmental sustainability.