As the global demand for sustainable practices intensifies, the field of chemical separation is witnessing novel solutions aimed at minimizing waste and enhancing efficiency. Traditional methods, often reliant on heat or mechanical filtration, frequently result in significant material waste and environmental concerns. However, the latest research from a team at the University of Illinois Urbana-Champaign introduces a groundbreaking approach using a polymer that selectively attracts particular substances when electrically activated. This innovative method could redefine how industries approach chemical separation, making it both efficient and environmentally friendly.
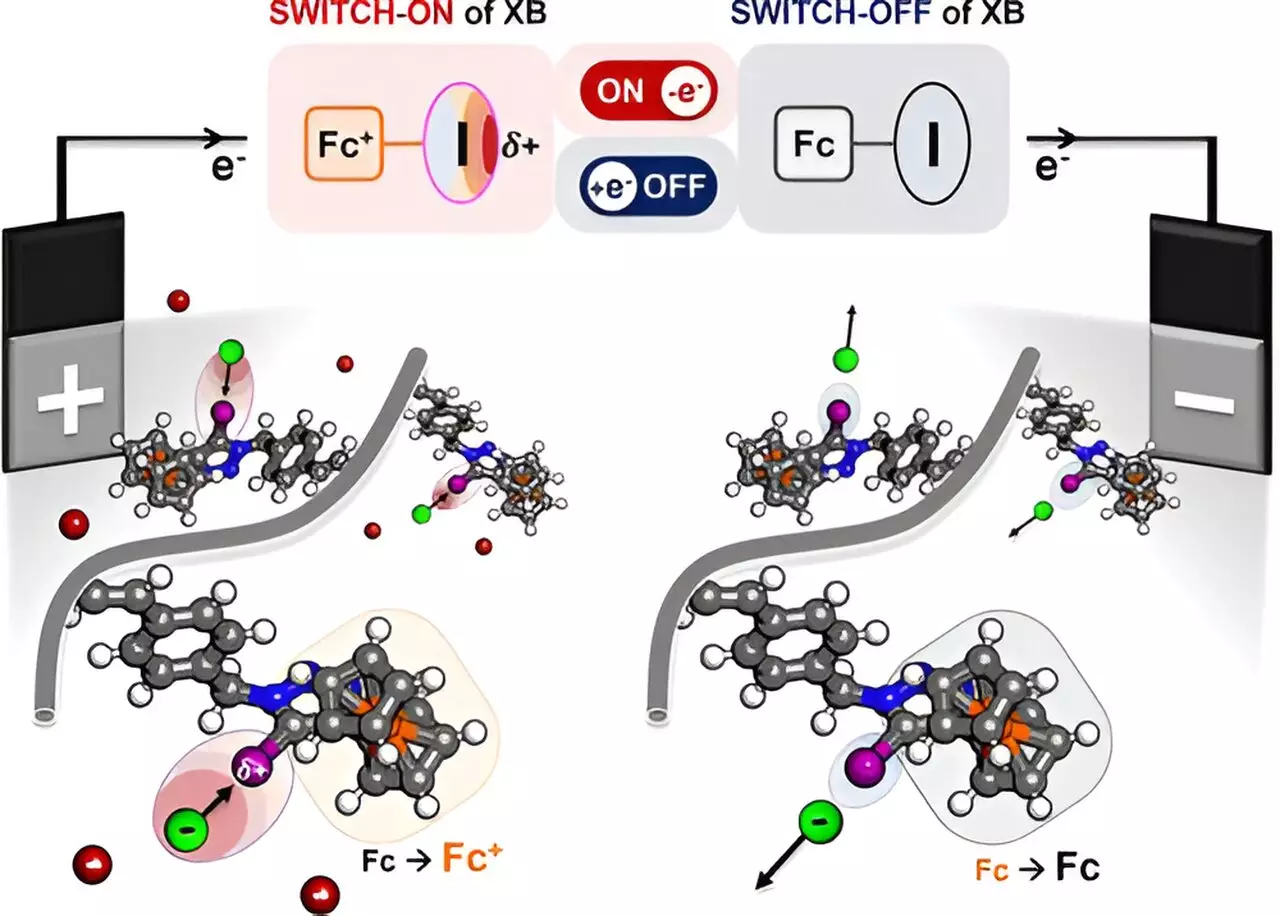
At the heart of this study lies the concept of electrochemical separation, traditionally viewed as a complex task due to its indiscriminate nature—the tendency of existing systems to attract a broad range of substances rather than specific targets. What sets this new polymer apart is its unique ability to selectively attract halides, oxyanions, and certain organic molecules through a process known as halogen bonding.
Halogen bonding typically involves the attraction between a positively charged region, referred to as a “sigma hole,” on a halogen atom and negatively charged ions. By engineering a polymer that can modify the charge density of the halogen atom in response to an electric current, researchers have essentially crafted a molecular “sponge” that selectively extracts desired compounds from complex mixtures.
The Role of Halogens and the Polymer Design
The research highlights the potential of halogen iodine within a redox-active polymer, paired with ferrocene—an electroactive component that can significantly alter the iodine’s bonding characteristics under electrical influence. Notably, when subjected to an electric current, the iodine atom’s bonding strength is optimized, enabling it to attract specific negatively charged ions with remarkable efficiency.
This clever design not only illustrates the inherent potential of halogen bonding but underscores a pivotal advancement in the scientific understanding of molecular interactions. The successful demonstration of this selective attraction is pivotal because it opens avenues for broader applications in pharmaceuticals, where precise chemical separations enhance product purity and efficiency in synthesis practices.
To confirm the effectiveness of the polymer, the researchers employed cutting-edge experimental methods such as nuclear magnetic resonance (NMR) and Raman scattering. These techniques allowed them to observe the selective interaction between the polymer and various ions, corroborating the polymer’s unique capability to function as a selective binding agent.
Collaboration with computational chemists further enriched the project by providing insights into the underlying mechanisms that govern the activation of the redox center, enabling an integrated approach to understanding both the theoretical and practical implications of the polymer’s design.
Future Directions and Potential Applications
The promising results from this research pave the way for future developments directed at optimizing and scaling the application of the selective electrochemical separation system. Plans are underway to refine the technology, including the development of continuous electrosorption systems that could operate beyond controlled laboratory conditions.
As scientists work towards creating more robust models, they aim to enhance the purity of the output through innovative strategies, such as the cascade separation model. This model would significantly improve efficiency, making the adoption of this technology in industrial settings more viable.
The pioneering work by the University of Illinois team not only emphasizes the potential of electrochemical systems but also represents a critical step forward in sustainable chemical practices. By designing an “electric sponge” that can selectively extract chemical substances, they are reshaping the landscape of chemical separation. The implications of their findings extend far beyond the laboratory, heralding exciting possibilities for industries reliant on efficient and environmentally friendly chemical processes. As the world increasingly shifts toward sustainability, such advancements could play a crucial role in both reducing waste and enhancing the efficiency of chemical production.