In the modern landscape of industrial chemistry, the emphasis on sustainable practices has become more pressing than ever. As the global community grapples with issues surrounding waste management and environmental health, the search for greener alternatives to conventional chemical processes is critical. Traditional methods predominantly rely on toxic organic solvents, which not only generate hazardous waste but also significantly impact the environment. Yet, a promising breakthrough emerging from India’s Indian Institute of Science (IISc) could transform the paradigm of chemical synthesis: a surfactant derived from agricultural waste that enables chemical reactions in water, significantly reducing reliance on harmful solvents.
Chemical industries regularly undertake the synthesis of diverse organic molecules, predominantly in liquid phases that facilitate interactions among substrates and catalysts. However, many of these critical chemical compounds and their catalysts show heightened sensitivity to water. This sensitivity often leads chemists to utilize toxic organic solvents, contributing to over 80% of waste produced in these processes. With improper disposal practices proliferating, the pressing need for sustainable alternatives has never been more pronounced. The challenges posed by traditional methods underscore the urgency for innovative solutions that not only minimize environmental footprints but also enhance the efficiency of chemical transformations.
Enter a team of researchers from IISc, who tackled this challenge by developing a novel surfactant known as CNSL-1000-M, harvested from cashew nut shell liquid (CNSL), an often-overlooked agricultural byproduct. This surfactant’s bio-based nature positions it as a greener substitute for conventional organic solvents, highlighting its potential in reducing the ecological impact of chemical processes. According to Pritesh Keshari, the initiative’s lead researcher, the accessibility of cashew nut waste in India—being one of the leading producers of cashews—makes CNSL-1000-M not only environmentally friendly but also economically viable.
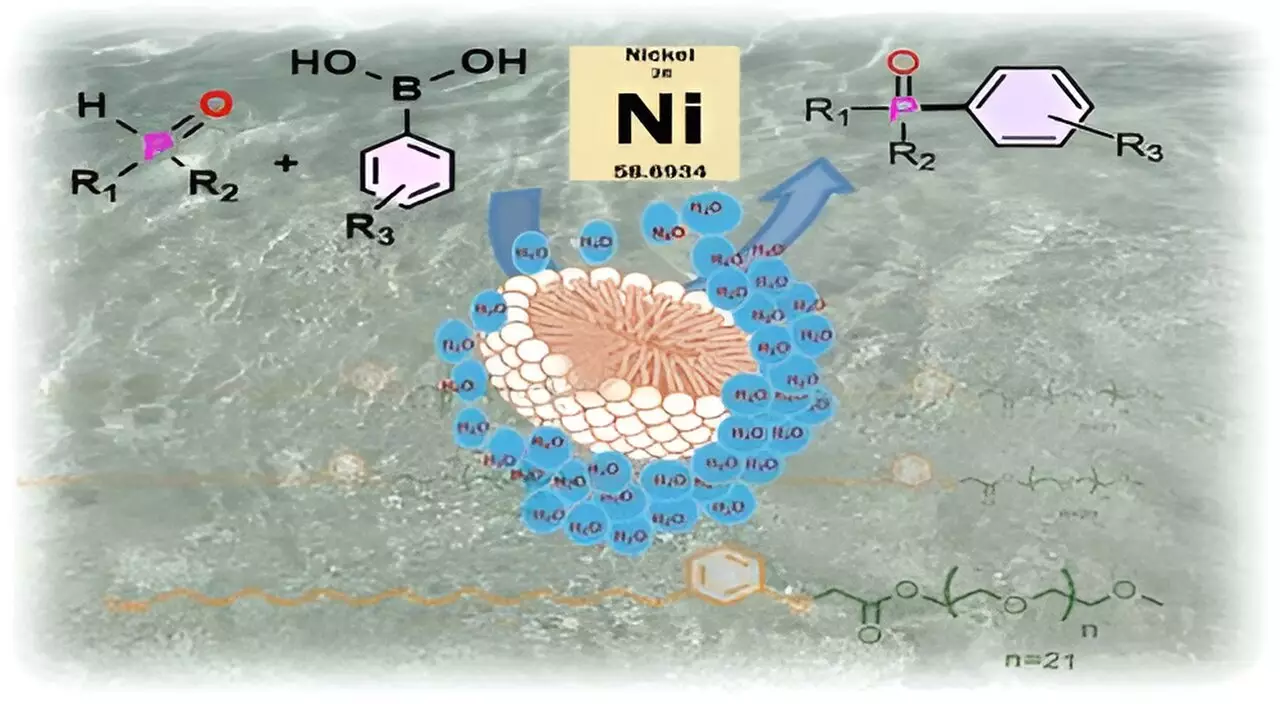
The significance of CNSL-1000-M lies not only in its source but also in its operational efficiency. Surfactants function by possessing both hydrophilic and hydrophobic attributes, enabling them to form micelles—structures that provide sheltered environments for sensitive substrates and catalysts. The assembled micelles create “pockets” away from the aqueous phase, protecting fragile compounds while enabling essential chemical reactions to occur. Susanta Hazra, another key researcher in the project, illustrates this concept by comparing it to a football floating in water; as long as the ball remains intact, it remains dry inside, akin to how micelles shield reactants from the surrounding water.
This innovative approach mirrors the natural design of enzymes, where hydrophobic pockets facilitate biochemical reactions, demonstrating a fantastic mimicry of biological systems. By embracing micellar catalysis, chemists can leverage nature’s solutions and apply them to industrial processes, exemplifying a transition towards greener chemistry.
The practical applications of CNSL-1000-M have proven significant. In trials focusing on the formation of carbon-phosphorus bonds—a chemistry pivotal in developing potential pharmaceuticals and electronic components—this bio-based surfactant exhibited an impressive 80% increase in product yield compared to traditional organic solvents. Furthermore, it outperformed existing surfactants by yielding 30% higher outputs in aqueous environments. Utilizing CNSL-1000-M also opens the door to replacing costly catalysts like palladium with more affordable materials such as nickel, while accommodating lower reaction temperatures.
The researchers’ ambitions extend beyond laboratory achievements; they are eager to foster collaboration with the chemical industry to facilitate the adoption of aqueous micellar technology. The promise of this sustainable method holds the potential to not only produce less waste but also to enhance the economic efficiency of chemical manufacturing processes.
As society moves further into an age where sustainability and environmental responsibility are vital, innovations like CNSL-1000-M embody the shift towards greener chemistry. This research illustrates an inspiring fusion of agricultural waste utilization with advanced chemical practices, paving the way for a more sustainable industrial future. Through continued exploration and collaboration, this remarkable approach could redefine chemical synthesis, steering the industry into an eco-friendlier direction and setting a precedent for further innovations. As we stand at the crossroads of science and environmental stewardship, endeavors like these will undoubtedly shape the legacy of modern chemical engineering.