Carboxylic acids undoubtedly hold a pivotal place in the realm of chemistry, underpinning countless organic synthesis processes and acting as essential building blocks for a wide array of pharmaceuticals. Substances such as aspirin and ibuprofen are just the tip of the iceberg when it comes to their applications in medicine. However, the quest to enhance the functional properties of these acids has led scientists to explore more complex structures through the introduction of fluorine atoms. While this advancement bears immense potential for the pharmaceutical industry, achieving it has historically been hampered by the intricacies of multi-step synthetic routes.
The Breakthrough in Direct Fluorination
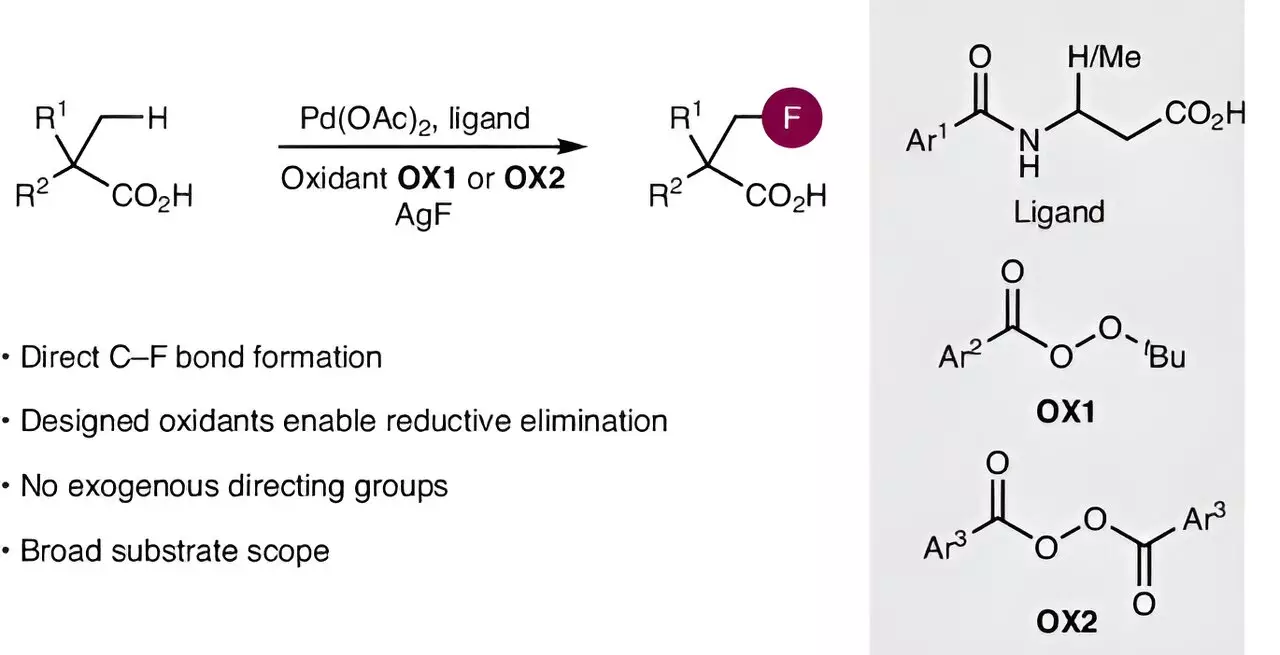
Recent revelations from a research team at the Otto Diels Institute of Organic Chemistry at Kiel University have illuminated a path forward that significantly eases the arduous process of fluorinating aliphatic carboxylic acids. Their pioneering work, published in the prestigious journal *Nature Synthesis*, showcases a novel methodology that brings efficiency to a field often characterized by cumbersome procedures. By harnessing the nearly unrealized potential of catalytic chemistry, this innovative method ushers in a new era of direct fluorination, transforming the way scientists can manipulate carbon structures.
Innovative Catalysts and Oxidants: A Game-Changer
At the heart of this transformative approach lies the activation of carbon-hydrogen (C–H) bonds, a notoriously challenging endeavor in organic synthesis. The palladium-based catalysts employed in this research signify a remarkable leap from previous attempts, as they enable functionalization of previously inert bonds. This catalytic breakthrough hinges on foundational work laid by various global research teams, including that of Professor Manuel van Gemmeren. Not only has this team refined existing catalysts, but they have also forged a new path by designing an oxidizing agent that plays a crucial role in forming carbon-fluorine bonds. This is particularly significant as traditional methods struggled to accomplish this in aliphatic carboxylic acids.
A New Paradigm for Synthetic Chemistry
What makes this research truly groundbreaking is its demonstration that combining distinct approaches—namely catalyst optimization and oxidant design—can yield results unachievable through conventional strategies. This insight opens up endless possibilities for other complex synthetic methodologies and invites chemists to rethink their strategies in molecular construction. According to Professor van Gemmeren, the findings not only streamline fluorination processes but hint at broader implications for synthetic chemistry at large, indicating a transformative wave of innovation on the horizon.
The Future of Pharmaceutical Applications
With carboxylic acids and fluorinated compounds being instrumental in pharmaceutical research, the potential application of this new method is nothing short of extraordinary. It implies a future where the synthesis of vital medications could be expedited, ensuring quicker access to effective treatments. This research marks a turning point that could revolutionize how we approach design and synthesis within the pharmaceutical industry, minimizing the time and complexity involved in drug development. As scientists continue to probe the depths of organic chemistry, this advancement stands out as a beacon of hope for researchers and pharmaceutical companies alike, emphasizing the importance of innovation in the chemical sciences.