In the realm of modern medicine, the ability to preserve sensitive biological materials is crucial for the advancement of healthcare. Treatments such as vaccines, blood products, and fertility preservation greatly depend on cryopreservation—the process of cooling and storing cells, tissues, or any other substances containing biological molecules at very low temperatures. This technique ensures the viability and efficacy of these materials over extended periods, allowing for a broader spectrum of medical applications. The significance of cryoprotectants, the molecules used to prevent cell damage during freezing, cannot be overstated; their role is essential in safeguarding these life-saving treatments.
Recent developments by a team of scientists from the University of Warwick and the University of Manchester have yielded a breakthrough computational framework for the discovery of effective cryoprotectants. Published in the prestigious journal *Nature Communications*, this innovative model enables researchers to test a plethora of new compounds virtually, significantly expediting the search for viable cryoprotectants. Through an intricate blend of machine learning and molecular simulations, this research moves away from traditional labor-intensive methods historically used in the selection and testing of cryoprotectants.
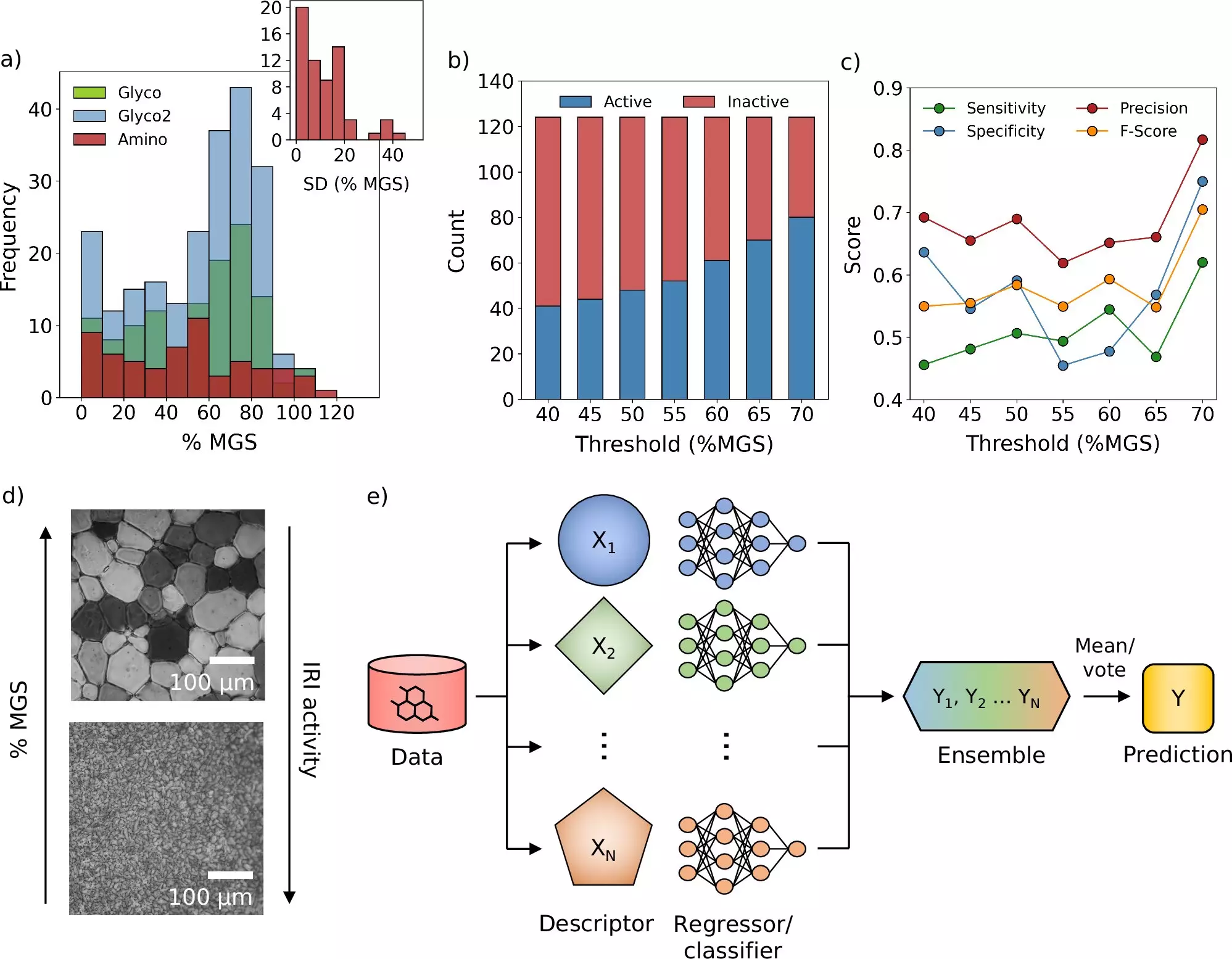
The computational model introduced allows researchers to explore substantial chemical libraries methodically, identifying those candidates that demonstrate the greatest potential in preventing ice crystal formation—a critical challenge in cryopreservation. Prof. Gabriele Sosso, who led this cutting-edge research, highlighted that while machine learning is a transformative tool, its true power lies in conjunction with experimental validation. This multifaceted approach stands as a testament to the evolution of research methods in scientific inquiry.
Ice crystal formation remains one of the most significant hurdles in cryopreservation. During the freezing process, if ice crystals grow uncontrollably, they can rupture cellular structures, thereby rendering the preserved material ineffective. Traditional cryoprotectants do offer some protection to cells but often fall short in halting the growth of these pesky ice crystals. The groundbreaking study tackled this issue head-on by discovering a novel molecule specifically designed to inhibit ice formation, thereby pushing the boundaries of what is possible in this vital area of healthcare.
Dr. Matt Warren, a Ph.D. student involved in the project, expressed his enthusiasm for how machine learning has taken over the repetitive and time-consuming tasks associated with data collection, thus allowing researchers to transcend routine experiments. This shift not only accelerates the pace of scientific discovery but also encourages researchers to delve into more complex challenges of a scientific nature.
The practical ramifications of this research extend into various avenues of medicine. In their experiments, the research team demonstrated that by incorporating the newly identified molecules, the amount of conventional cryoprotectants required for blood storage could be minimized. The potential to expedite blood transfusions provides an immediate benefit that could save lives in critical situations. Moreover, the successful integration of machine learning into this field foreshadows an era where scientific exploration could witness unparalleled acceleration, innovation, and collaboration.
Through the collaboration of different experts, including insights from Prof. Matthew Gibson, who specializes in ice-binding proteins, additional opportunities for research have emerged. By synthesizing knowledge from various domains, the project aims to uncover alternative uses for existing compounds, speeding up the optimization of cryoprotectants in real-world applications.
As we stand on the cusp of a new era in cryopreservation research, the framework developed by this collaborative team heralds a transformative shift in medical science. By reducing reliance on time-consuming traditional methods and empowering discovery through machine learning-backed models, the door opens to novel treatments that could redefine the limitations of cryogenic storage. With the ongoing commitment to scientific innovation, the landscape of medicine appears poised for unprecedented advancements that will enhance both the effectiveness and availability of critical health treatments.