As the world grapples with an escalating energy crisis, hydrogen energy emerges as a beacon of hope, boasting properties that are green, low-carbon, and highly calorific. With the growing urgency to transition to renewable energy sources, hydrogen stands out as a potential game changer. It promises not only to fuel vehicles but also to power entire industries without the harmful emissions associated with fossil fuels. However, realizing the full potential of hydrogen as a clean energy carrier hinges on overcoming significant technical challenges in its production.
Among the various methods of hydrogen generation, electrochemical water splitting has attracted considerable attention. This process involves using electrical energy to break down water into hydrogen and oxygen, a reaction that sounds straightforward but presents intricate scientific challenges. A critical hurdle remains in the form of the oxygen evolution reaction (OER), which takes place at the anode and is notoriously slow. This inefficiency severely restricts the overall energy conversion efficiency of the reaction, highlighting the urgent need for innovative solutions.
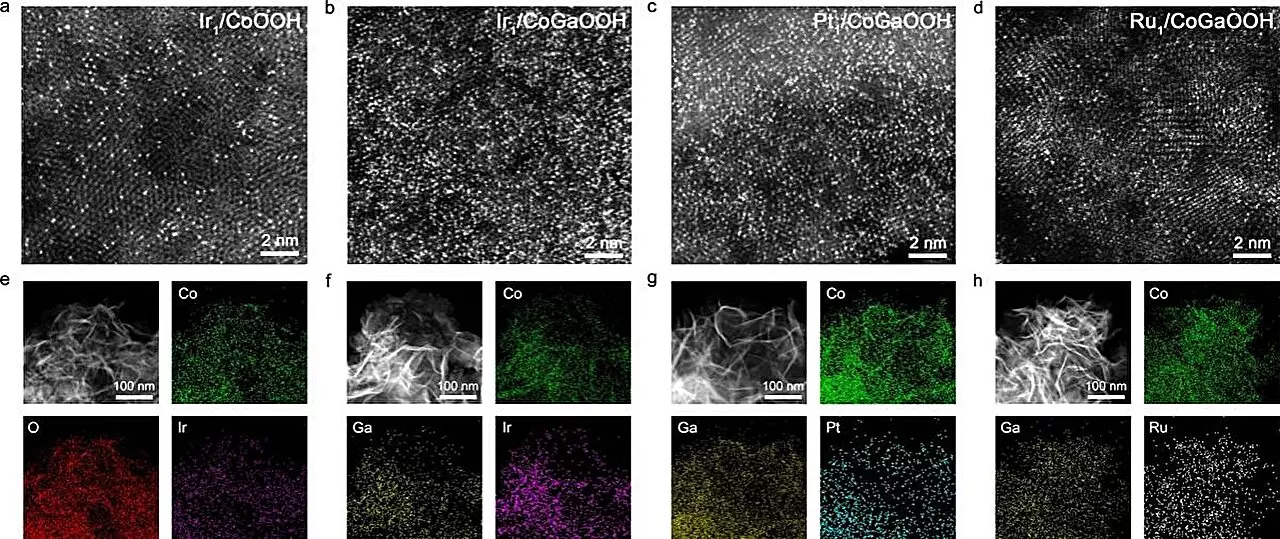
To enhance the performance of the OER, significant research has been directed toward the development of efficient catalysts. Recent advancements have showcased the importance of single-atom catalysts (SACs), which consist of isolated atoms dispersed across a substrate. Sac-managed reaction sites are a promising direction; however, the actual performance of these catalysts hinges significantly on the density of the active single atoms. The more closely placed these atoms are, the more likely they are to interact synergistically, leading to better catalytic performance.
A notable breakthrough in this domain has been achieved by a research team at the University of Science and Technology of China, led by Professor Bao Jun. Their study, recently published in *Angewandte Chemie*, presents a cobalt-based, oxide-supported high-density iridium (Ir) single-atom catalyst. This innovative catalyst exhibits remarkable electrochemical performance during the OER, driven by the synergistic effects of neighboring single atoms. The team’s work expands the understanding of how atom density and proximity can optimize catalytic activities, providing a foundation for future research.
Synthesis Strategies: Modulating Atomic Density
To craft these high-density single atoms, the researchers employed a novel approach by incorporating gallium (Ga) atoms into the cobalt-based oxide lattice. This modification adjusted the electronic structures of the anchoring sites for the single atoms, intensifying the bond strength with oxygen defect sites. This strategic enhancement not only led to the construction of a series of high-density SACs but also facilitated the exploration of their performance in the OER.
The resultant catalyst, known as Nei-Ir1/CoGaOOH, demonstrated promising results, achieving a low overpotential of 170 mV at a current density of 10 mA cm^-2. Moreover, it exhibited impressive stability, withstanding operational conditions for over 2000 hours. Even more striking, this catalyst could maintain stability at a current density of 1 A cm^-2 for over 50 hours in alkaline electrolytes, indicating its practical viability for large-scale applications. The results clearly illustrate not only the capability of these new catalysts but also their potential influence on the industry.
Understanding Performance: The Role of Synergistic Interactions
Further mechanistic investigations revealed that the superior performance of the high-density Ir single-atom catalysts stems from the neighboring synergistic interactions rather than solely from optimized electronic structures. These interactions stabilize reaction intermediates, specifically *OOH*, through additional hydrogen bonding. This stabilization effectively lowers the energy barrier associated with the reaction, further enhancing catalyst performance. This understanding paves the way for future efforts in catalyst design aimed at maximizing those beneficial atomic interactions.
The research conducted by Prof. Bao Jun and his team sheds light on the pivotal role that high-density single-atom catalysts can play in advancing electrochemical OER technology. By unveiling the mechanisms behind their enhanced performance, this study not only contributes to the ongoing discourse on hydrogen energy as a sustainable solution but also offers valuable insights for future innovations in catalyst fabrication. As the world seeks to transform its energy landscape, such advances may be crucial to the development of efficient, renewable hydrogen production techniques.