Ice, though often viewed as a mere solid frozen form of water, is a fascinating subject of study, particularly when considering its interaction with liquid water. Nature’s intricate dance between ice and liquid is omnipresent, from the frost on winter mornings to the soothing sensation of cold ice cream on a hot day. Understanding how these two states of water interact is not just an academic curiosity; it has far-reaching implications for various scientific fields, including climate science, material science, and even culinary arts. The recent study led by Kobe University and the Institute for Molecular Science offers new insights into this dynamic relationship by employing innovative techniques to visualize the ice-water interface.
Innovative Methods to Explore the Ice Landscape
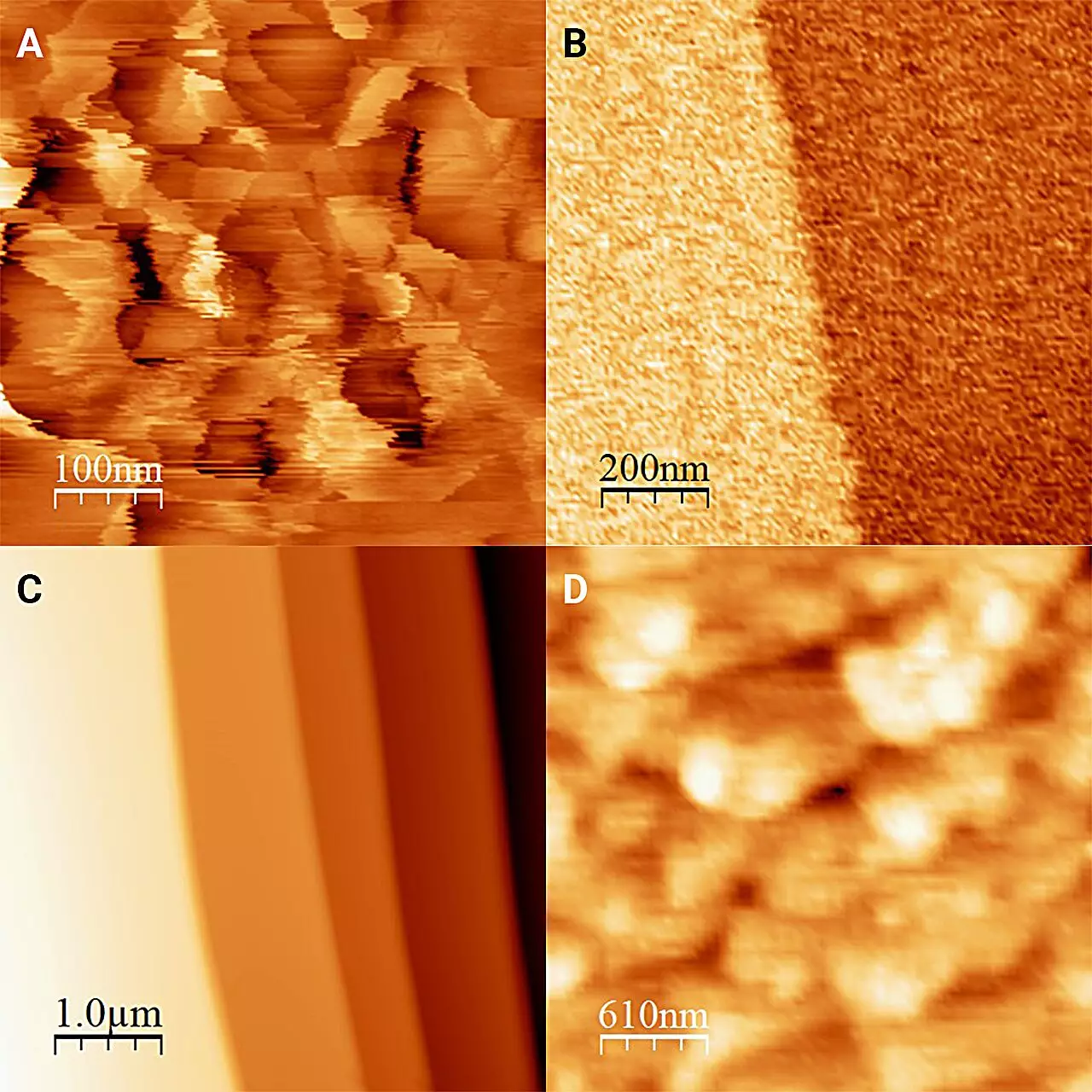
The primary challenge in researching ice at such interfaces has always been its tendency to transition rapidly between solid and liquid states. Conventional methods lacked the precision necessary to observe these fleeting moments. Embracing a novel approach, the researchers utilized antifreeze and a specialized refrigerated microscope to create a stable environment for their observations. By immersing ice in a solution colder than 0°C but preventing it from melting, the team achieved the delicate balance necessary to capture the interaction between the solid and liquid phases.
Kobe University’s Onishi Hiroshi and his colleagues encountered significant hurdles during their experimentation. The task of cooling the entire microscope system encapsulated in a cooling box was no small feat. The need for precision instruments, such as the atomic force microscope, to operate under these sub-zero conditions required creativity and persistence. Their triumphs in overcoming these technical barriers are a testament to the importance of innovative thinking in scientific research.
Unveiling New Dimensions of Ice Structure
The findings published in The Journal of Chemical Physics reveal fundamental differences between ice in the absence of liquid and ice in contact with antifreeze. The researchers discovered that ice exposed to a liquid medium exhibits a significantly flatter surface than ice in dryer conditions, where it forms distinctive “frost pillars” measuring approximately 20 nanometers in height. This flattening effect is attributed to processes of partial dissolution and recrystallization, demonstrating how ice surface characteristics can radically transform depending on the immediate environment.
Moreover, the study underscores the diverse responses of ice to various liquid media. While other alcohols tested showed similar properties, the distinct microscopic appearances of the ice’s surface across these mediums highlight the intricate details occurring at the molecular level. Such variability prompts crucial questions about the profound impacts that different liquids can have on ice’s physical properties.
Reevaluating Ice’s Mechanical Properties
One of the standout revelations of this research is the newfound understanding of the hardness of ice surfaces in contact with liquid. The investigation into how the ice’s surface behaves under 1-octanol revealed that its hardness extends beyond previous estimates derived from less direct measurement methods. This newfound toughness indicates that ice, often regarded as a fragile solid, possesses robust mechanical properties that merit further exploration.
The implications of these findings extend to several domains. For instance, in a world increasingly affected by climate change, understanding how ice responds to temperature variations and changes in liquid states could provide insights into the melting of glaciers and polar ice caps. Additionally, in various industrial applications where ice is used as a coolant or in manufacturing processes, recognizing the structural characteristics of ice can enhance the efficiency and safety of operations.
A Call to Further Exploration
The research team at Kobe University is not resting on their laurels. They anticipate that their ground-breaking findings will pave the way for additional studies focused on the ice-liquid interface, with aspirations to further enhance the resolution of their measurement techniques. The ambition to observe single water molecules at the ice surface indicates a frontier of research that could uncover even more phenomena of this omnipresent substance.
In this context, studying the interplay between ice and liquid is more than merely academic; it’s a complex narrative with environmental, industrial, and culinary chapters yet to be written. As scientists build upon these foundational insights, we can look forward to a greater understanding of ice in nature, which will indisputably enrich our grasp of the world.