Photocatalysis is a process in which light energy drives a chemical reaction. Stemming from nature’s photosynthesis, it offers a sustainable alternative to traditional high-temperature reactions. This technology has gained traction as researchers tirelessly pursue ways to enhance the efficiency of these reactions, ideally making them economically viable for widespread industrial application. Central to the success of photocatalysis is the quantum efficiency of the light-induced transformations, which needs to be optimally elevated to realize its full potential.
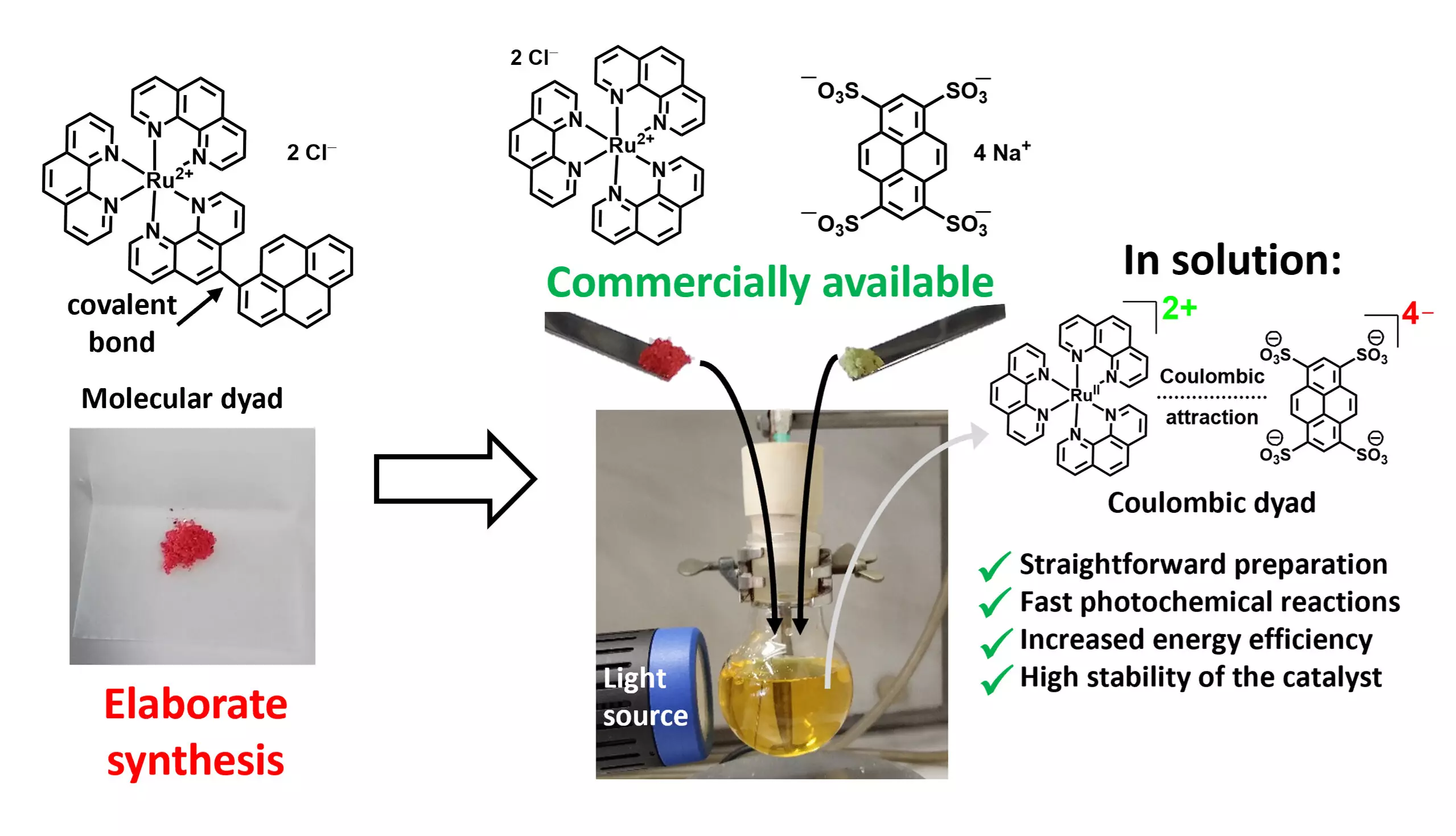
The concept of using tailored photocatalysts—particularly molecular dyads composed of two photoactive units linked by a covalent bond—has become a focal point of research. However, the multi-step synthesis required for creating these dyads presents significant challenges, particularly in terms of cost, making them impractical for large-scale application.
In a significant stride toward overcoming the limitations of traditional photocatalyst production, a research team led by Professor Christoph Kerzig at Johannes Gutenberg University Mainz has introduced an innovative method for synthesizing highly efficient dyad photocatalysts. Their approach leverages the interaction of commercial salts, wherein electrostatic (Coulombic) forces facilitate the formation of ion pairs. This revolutionary mechanism promotes a synergistic interaction between the photoactive units, thereby expediting the photocatalytic process while averting the complexities of prior multi-step synthesis.
Matthias Schmitz, the lead author of this pivotal study, elucidates the simplicity of their method by drawing a parallel to table salt, where sodium and chloride ions exhibit attractive forces that stabilize each other. The research, published in the esteemed Journal of the American Chemical Society, emphasizes a shift towards more accessible raw materials and methodologies in photocatalysis.
Currently, scientific explorations are engaged in reconditioning non-precious metals to mimic the behavior of traditional catalysts, such as those based on rare elements like iridium and ruthenium. These sophisticated methods often involve labor-intensive synthesis of complex ligands, further complicating the scalability of such catalysts. However, Kerzig’s method shines a light on a different path—enhancing established photocatalysts by integrating inexpensive additives, thus doubling their effectiveness without the burden of elaborate preparation.
This strategy has profound implications for the field, as it allows researchers to optimize the use of metal-based photocatalysts more efficiently. By significantly reducing the requisite amount of catalyst, it paves the way for more sustainable chemical processes.
A noteworthy component of the Kerzig research group’s approach involves the use of spectroscopy-guided optimization. By employing advanced laser systems, they meticulously dissect each reaction phase—from light absorption to the eventual activation of the photonic energy-storing molecules. This precision-guided methodology ensures that the insights gained can be directly translated into practical applications.
Initial experimental trials with the Coulombic dyad catalysts have produced promising results. The team explored reactions that create new carbon-carbon bonds and conducted photooxygenation of biomass-derived materials. The efficacy of the newly synthesized catalysts exceeded that of traditional and usually pricier alternatives, confirmed by their ability to harness sunlight and LED light more effectively for chemical transformations.
The astounding initial results underscore the potential of the Coulombic dyad concept in photocatalytic research. The researchers contend that the solvent used during the reactions plays an integral role in optimizing the outcome, allowing for a toolbox approach wherein different photoactive ions can be mixed and matched. This adaptability signifies a leap toward customizable, efficient photocatalytic reactions that can be fine-tuned for various industrial applications.
As the scientific community shifts its focus to sustainable practices, the implications of the Kerzig team’s findings could be vast and transformative. Their research not only evolves the landscape of photocatalysis but also opens pathways toward large-scale utilization of these innovative photocatalysts, reflecting a promising future for green chemistry and industrialized photochemical processes. Through further exploration and optimization, the potential for revolutionizing chemical processes in an environmentally sustainable manner becomes increasingly attainable.