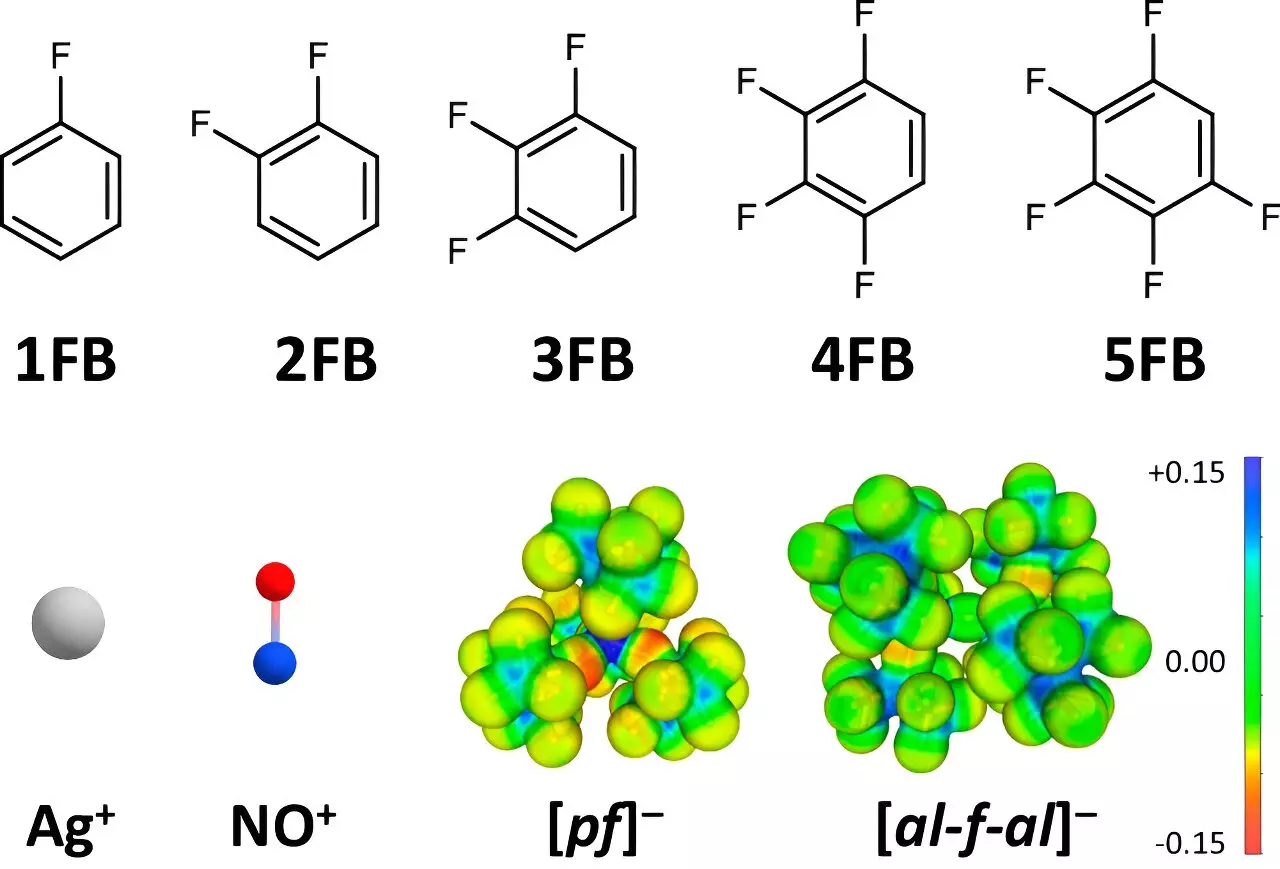
Recent developments in molecular and coordination chemistry have opened up new avenues for the study of positive ions, notably through the research conducted by a team led by Professor Ingo Krossing from the University of Freiburg. Their findings, which have been documented in *Nature Communications*, underline a pivotal shift in our understanding of oxidation potentials in various chemical environments. Traditionally, the maximum oxidation potentials for well-known positive ions such as Ag+ and NO+ were limited to approximately +0.65 to +1.0 V compared to the ferrocene (Fc+/0) standard. This new research pushes those boundaries significantly, achieving oxidation potentials of up to +1.52 V.
This innovative advancement primarily stems from the employment of weakly interacting solvents and tailored anions that minimize undesired interactions between positive ions and their environments. The implications of this research stretch beyond theoretical chemistry and delve into practical applications across multiple fields, including electrocatalysis and the manipulation of redox mediators.
The researchers strategically utilized fluorinated benzene derivatives as solvents due to their unique properties. When examining the characteristics of these compounds, Dr. Johannes Hunger from the Max Planck Institute for Polymer Research contributed critical data on dielectric constants as they relate to solvent behavior. The analysis revealed that the synthesization of two to four fluorinated aromatic compounds led to significantly higher dielectric constants compared to conventional solvents like dichloromethane and acetone. These insights demonstrate that while conventional solvent systems exhibit strong attractive forces towards positive ions, the increased fluorination effectively reduces these interactions, allowing for enhanced oxidation capability of the dissolved ions.
This discovery fundamentally alters the landscape of reactions involving these positive ions, allowing for previously unattainable redox reactions. The weakly coordinating properties of the anions and solvents facilitate an environment where oxidation can proceed more freely, making it a game-changer for numerous chemical applications, particularly in areas known for challenging oxidation processes.
Another critical component of this research involved elucidating the structural relationships between the solvents and the positive ions through single-crystal X-ray diffraction techniques. Co-author Dr. Malte Sellin noted that the extent of fluorination in these aromatic compounds correlates inversely with the strength of their interaction with Ag+ and NO+ ions. The structural evidence provided by X-ray diffraction not only supports the electrochemical findings but also demonstrates an unprecedented level of oxidation potential that was previously unattainable.
These insights open up numerous possibilities for exploring new chemical pathways and reactions. By lessening the environmental interaction, the ions can exhibit higher reactivity in various applications—from advancing electrocatalysis to creating more efficient redox shuttles.
The implications of this research are wide-ranging, as they promise to pave the way for transformative studies in elementary chemistry. As researchers harness the potential of weakly coordinating solvents and tailored anionic environments, we can anticipate a surge in innovative methodologies for exploring oxidation processes. The enhanced potentials signify that even previously “hard-to-oxidize” systems may now become viable candidates for study.
The work spearheaded by Krossing and his team hints at a remarkable trajectory in the field of coordination chemistry. The ability to achieve higher oxidation potentials through strategic solvent and anion selection not only redefines our current understanding but also encourages future exploration into novel chemical applications. The scientific community stands at the precipice of new discoveries that can reshape the foundations of chemical synthesis and catalysis, risking the familiar in pursuit of the extraordinary.