Microplastics are becoming an omnipresent threat to ecosystems, especially water bodies, as they infiltrate freshwater lakes and rivers worldwide. These tiny plastic particles, often less than five millimeters in size, originate from a variety of sources, including the degradation of larger plastic items, industrial waste, and personal care products. A recent study highlights how environmental conditions, such as freezing and melting cycles, play a critical role in the behavior and fate of microplastics in aquatic environments.
Microplastics have a dual nature concerning their buoyancy and stability. Different types of polymers exhibit varying characteristics when introduced into aquatic systems—some float, while others sink based on their density. For instance, polyethylene (PE), a common plastic, is lighter and tends to stay afloat. In contrast, more substantial polymers like polyurethane (PU) and polytetrafluoroethylene (PTFE) typically sink to the bottom. This behavior is crucial in understanding how microplastics impact water quality and sediment dynamics, making it essential to explore environmental factors that exacerbate or mitigate their effects.
The recent research published in *Environmental Science & Technology* delves into the less-studied impact of freezing temperatures on microplastic behavior. The researchers, led by Chunjiang An, conducted experiments simulating various aquatic environments to analyze how freezing influences microplastic particles of PE, PU, and PTFE. By examining these particles before and after a freeze-thaw cycle, the study reveals critical insights into how seasonal changes may alter the ecological footprint of microplastics.
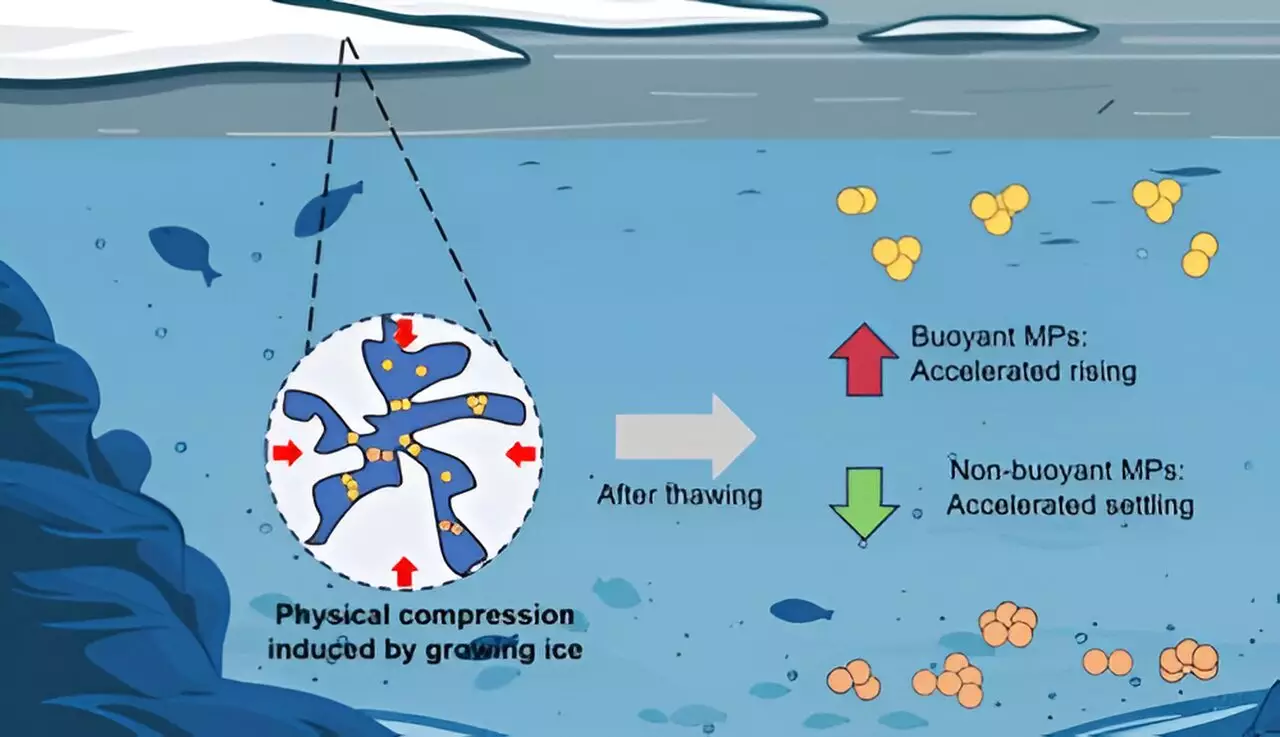
During the freeze-thaw experiment, the researchers observed a size increase in microplastic particles when exposed to freshwater conditions. The study noted that PE particles displayed a significant size increase of approximately 46%, while PU particles only experienced a modest 9% growth. The research team proposed that the differences in particle behavior could be attributed to how these polymers interact with water—hydrophobic PE particles repel water, leading to a clumping effect, while hydrophilic PU particles attract water, facilitating better dispersion among the water column.
Intriguingly, as salinity levels rose in the experiment, the size increase of the microplastic particles following freezing was negligible. This finding suggests that in saline environments, brine channels formed within ice might prevent particles from merging into larger aggregates, maintaining their distribution in the water column and potentially affecting their eventual sedimentation.
The physical forces at play in aquatic environments are critical to understanding how microplastics distribute and settle. The study employed mathematical models to evaluate gravitational, buoyant, and drag forces acting on microplastic particles post-freezing. The results indicated an increased buoyancy force acting on PE particles, empowering them to rise more quickly, while denser PU and PTFE particles became more likely to sink at an accelerated rate.
This revelation underscores a significant concern: as more microplastics settle in sediments due to seasonal freezing, they may contribute to the long-term pollution of aquatic ecosystems. The study emphasizes that the 24-hour freezing period tested in the lab is considerably shorter than natural freezing cycles, which could last for months or years, further complicating the dynamics and potential impacts of microplastics in colder regions.
The findings from this research bear considerable implications for policymakers and environmental managers. Understanding the impact of seasonal changes on microplastic behavior can inform strategies to mitigate pollution levels in aquatic environments, especially as climate change continues to influence temperature and precipitation patterns.
Future research should explore longer freeze-thaw cycles and their cumulative effects on various polymer types, seeking to provide a clearer picture of microplastic behavior under diverse and prolonged environmental conditions. As we move forward, a multidisciplinary approach combining ecology, materials science, and policy will be essential in addressing the multifaceted challenges presented by microplastics in our ecosystems.
As we continue to confront the pervasive issue of microplastic pollution, studies like this one remind us that our ecosystems are far more intricate than we tend to acknowledge, with seasonal changes shaping the ecological dynamics of our water bodies in ways we are only beginning to understand.