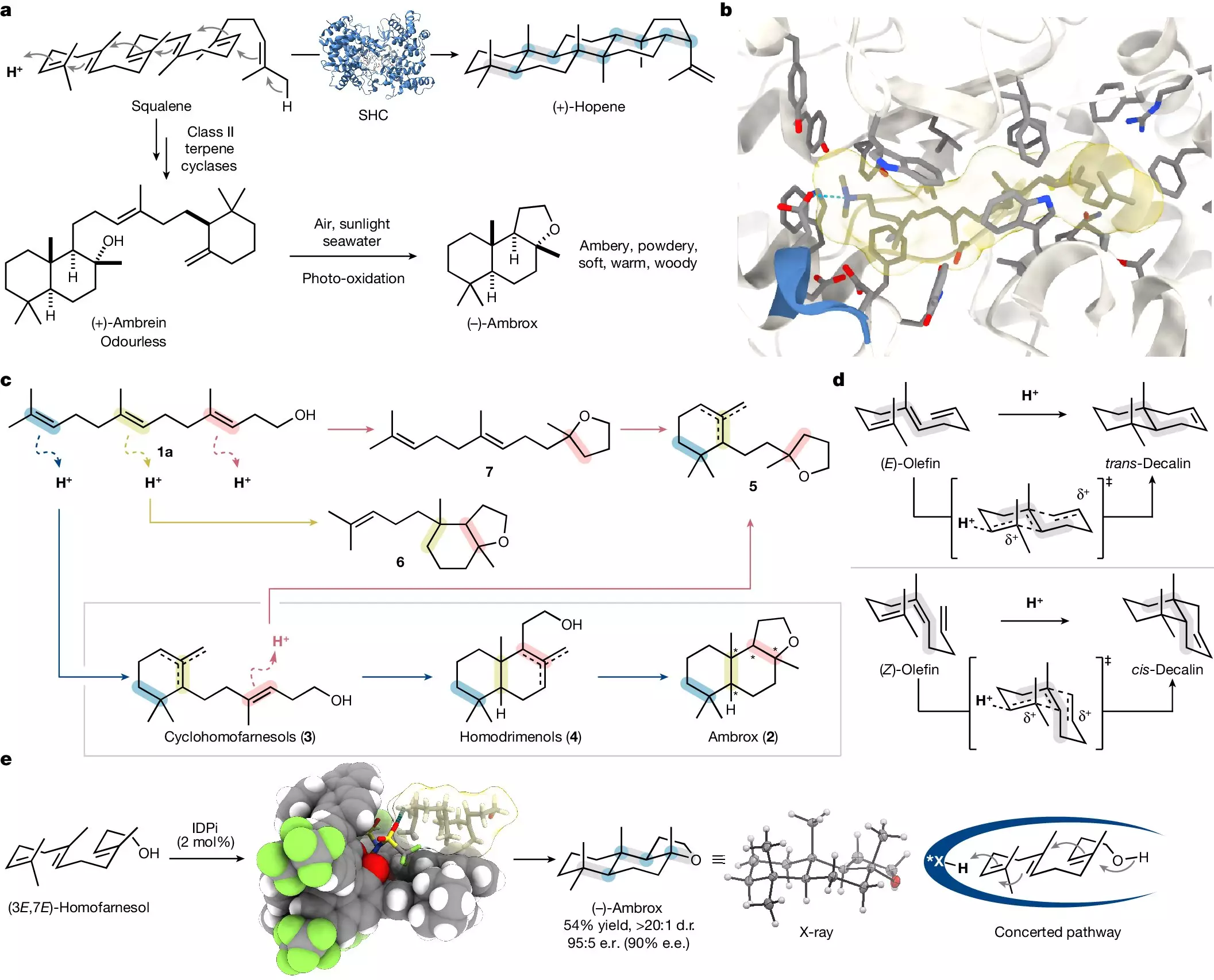
Humankind’s relationship with scent dates back to ancient civilizations, where enchanting aromas were not only prized for their pleasing qualities but were also thought to convey health and vitality. The pursuit of perfumes has evolved over the centuries, transitioning from the use of rare and elusive natural sources to contemporary scientific approaches. The fragrance industry is a testament to this shift, with compounds like ambrox—traditionally sourced from ambergris, which originates from the digestive systems of sperm whales—highlighting both the allure and complexity of perfume creation. Today, ambrox remains a highly sought-after ingredient, with approximately 30 tons produced annually, but the methods of obtaining it have seen substantial innovation.
At the heart of ambrox synthesis lies a fascinating scientific puzzle: the chiral nature of the compound. Chiral molecules exist in multiple forms—referred to as enantiomers—that often exhibit vastly different properties. Ambrox has over a dozen possible configurations, yet only one variation produces the beloved scent curated by perfumers worldwide. As a result, developing a synthesis method that selectively yields this specific enantiomer becomes essential. Advances in asymmetric synthesis—specifically stereoselective synthesis—have paved the way for more sustainable methods of production that no longer rely on the laborious and ethically questionable sourcing from marine animals.
Emerging Synthetic Pathways
Fortunately, researchers like Prof. Benjamin List and his team at the Max Planck Institute for Coal Research have taken strides in creating laboratory synthesis methods that are both efficient and ethical. Their innovative approach involves synthesizing ambrox from the natural compound (-)-sclareol, derived from clary sage, thus reducing dependence on scarce marine resources. However, as clary sage availability fluctuates, the team sought alternative pathways. Their work is reflective of a broader trend within chemistry—an aim to mirror nature’s complexity with the simplicity of synthetic processes.
In their groundbreaking research, recently published in the journal *Nature*, the List group introduces a catalytic technique that simplifies the conversion of homofarnesol, a compound sourced broadly from plants and easily synthesized, into the desired ambrox. The process draws inspiration from biological mechanisms, aiming to replicate nature’s own efficient pathways. This pursuit showcases the impressive intersection of chemistry and biomimicry, emphasizing not only the importance of methodology but also the underlying philosophy of research that seeks to learn from nature’s intricate designs.
One notable achievement of the List laboratory’s process is its high level of efficiency. While conventional biocatalytic techniques could necessitate several days for completion, the new synthetic method achieves the same outcome in a matter of hours, operating under relatively mild conditions. The utilization of a unique catalyst alongside a specially formulated fluorinated solvent enhances both the reaction speed and selectivity. Such innovations are critical for scalability, promising applicability in industrial settings where speed and cost-effectiveness play vital roles.
Another significant aspect of this advancement is the recyclability of both the catalyst and solvent, promoting a sustainable approach to chemical manufacturing. This characteristic aligns with current global trends emphasizing eco-friendly practices and the need to minimize waste—a conscious effort that benefits both the environment and industry stakeholders.
As the world moves towards more sustainable practices, the research led by Prof. List exemplifies how advanced chemistry can unlock new potential within traditional industries. The laboratory synthesis of ambrox presents an exciting prospect, not only for perfumers but also for the broader realm of synthetic biology and materials science. By effectively mimicking nature’s pathways while enhancing efficiency, chemists are paving the way toward a future where the allure of fragrance is attainable without compromising ethical standards or environmental sustainability. This research stands as a beacon of innovation, reminding us that the thirst for beauty and inspiration in scent can indeed be quenched through thoughtful scientific inquiry.