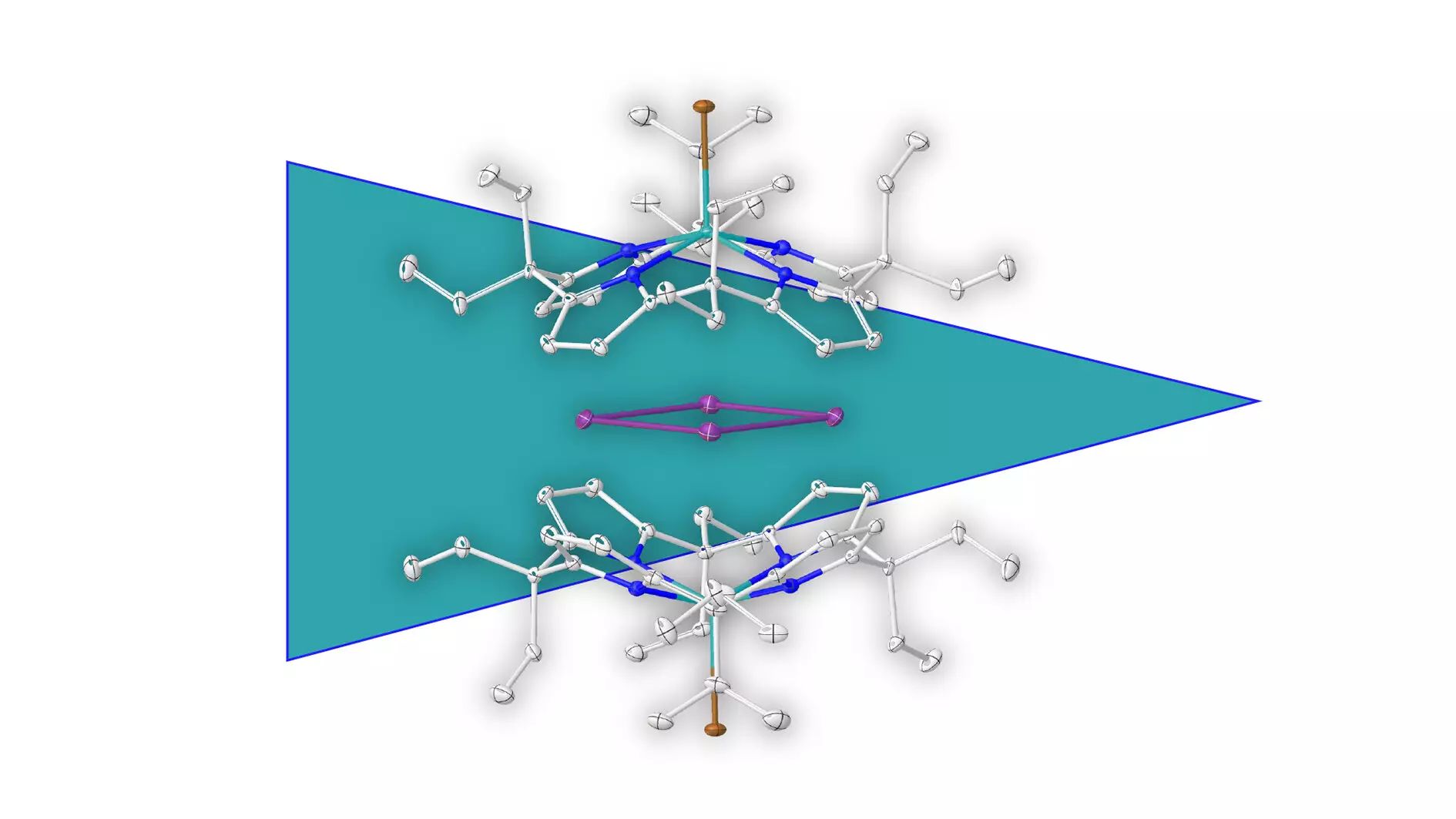
Aromaticity has long been the cornerstone of organic chemistry, primarily associated with the classic ring structures formed by carbon atoms. This concept not only helps to understand molecular stability and reactivity in carbon-based compounds but also lends itself to the aromaticity of various chemical entities. However, the recent breakthrough of isolating an aromatic ring consisting entirely of metal atoms shakes the very foundation of what we thought we knew about this essential aspect of chemistry. Conducted at Heidelberg University under the guidance of Prof. Dr. Lutz Greb, this pioneering research is set to revolutionize our insights into both organic and inorganic chemistry.
Understanding the Unprecedented Discovery
For decades, scientists have acknowledged the aromatic properties of metal complexes, which typically feature a metal atom attached to an aromatic organic molecule. The research team has now boldly ventured into uncharted territory by unveiling a metal ring composed entirely of elemental bismuth. This innovative leap in chemical inquiry opens new avenues for exploration in the realm of metal aromaticity—an area previously considered non-existent. Through meticulous experimentation, the team has successfully isolated and characterized this metal ring, a milestone that is sure to shift paradigms within the scientific community.
The Magic of Supramolecular Stabilization
A pivotal aspect that enabled this breakthrough is a novel approach revolving around supramolecular stabilization. The researchers ingeniously developed a negatively charged molecular shell that envelops the positively charged metal ring. This arrangement effectively safeguards against potential decomposition, illustrating the power of strategic molecular design in stabilizing unconventional aromatic systems. Greb emphasizes that this methodology may pave the way for broader applications in stabilizing other positively charged rings and cages, reflecting an optimism that resonates throughout the field of molecular chemistry.
Implications for Charge Transport and Beyond
The ramifications of this research extend far beyond the pure theoretical implications. Greb speculates that these newly minted aromatic compounds of solely metal atoms could have significant implications for charge transport in metals, thus opening up potential applications in fields such as electronics and materials science. Understanding the behavior of these unique metal rings may provide insights that could lead to more efficient conductive materials and novel electronic devices, which are crucial in our technologically driven world.
A Gateway to Future Research
The discovery exemplifies how pushing the boundaries of traditional chemistry can yield unexpected new avenues for research. As scientists continue to probe the depths of aromaticity, the existence of metal-only aromatic compounds posits exciting challenges and inquiries about elemental interactions and stability. It beckons other researchers to re-evaluate aromatic principles not as confined to carbon structures but as a universal trait that could encompass diverse elements, thus enriching our overall understanding of chemical behavior and interaction.
This progressive viewpoint is more than mere optimism; it indicates a pivotal shift in chemical discovery, charting a course for inquisitive minds eager to explore the multifaceted nature of aromaticity beyond traditional boundaries.