Recent advancements in chemical engineering have unveiled a pivotal breakthrough in the conversion of carbon dioxide (CO2) into valuable resources. A research team from the University of Twente, spearheaded by Georgios Katsoukis, has discovered the significant impact of the chemical environment surrounding copper electrodes on the reduction of CO2 to formate. This finding not only propels our understanding of electrochemical processes but also presents an opportunity to enhance the efficiency of CO2 reduction technologies that are vital in combating climate change.
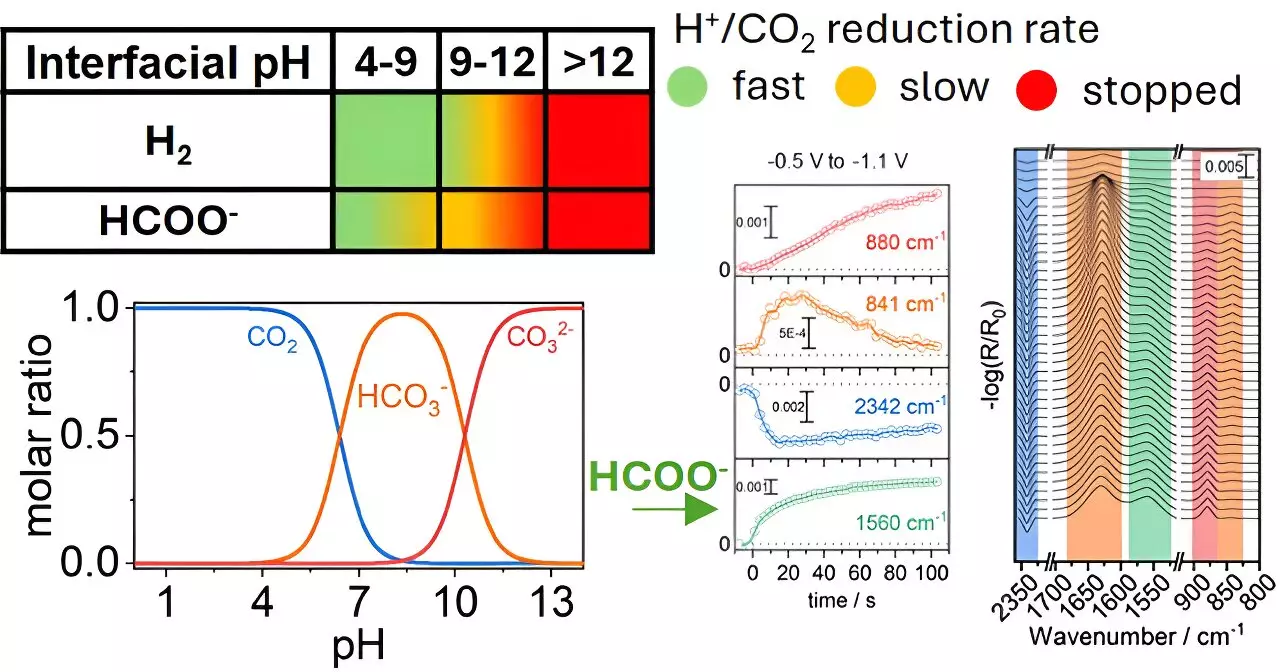
The study published in ACS Catalysis emphasizes the importance of the local chemical environment during the electrochemical reduction process. The researchers identified that varying the pH level near copper electrodes plays a critical role in how CO2 is converted into formate—a compound that serves multiple industrial purposes. Through a systematic examination, the team uncovered that a well-optimized chemical environment can dramatically increase the rate and efficiency of CO2 conversion. Rather than focusing solely on the inherent properties of the catalyst—copper in this case—this research highlights the necessity of considering surrounding conditions as a significant factor in the overall reaction.
One of the most enduring challenges in CO2 reduction is achieving selectivity, as unwanted by-products can emerge depending on reaction conditions. Traditionally, the focus has been on improving the catalysts themselves, but Katsoukis and his team underscore a paradigm shift: to improve selectivity, one must optimize not just the catalyst material but also the chemical milieu in which reactions occur. This study challenges previous notions and calls for a comprehensive approach to selectivity, opening avenues for new experimental designs.
The implications of this research extend beyond academic interest; they are crucial for sustainable development and the promotion of a circular economy. By effectively capturing CO2 emissions and converting them into usable products, society can mitigate greenhouse gas effects while simultaneously creating valuable resources. Enhanced CO2 reduction technologies can lead to the formation of sustainable systems that contribute to economic growth.
Katsoukis’s findings provide a compelling blueprint for future research endeavors in CO2 reduction technologies. By prioritizing the optimization of both the catalyst and its surrounding environment, scientists may develop more efficient methods for CO2 conversion. This integrated approach may pave the way for practical applications, making strides towards realistic solutions for carbon capture and utilization.
This innovative exploration of copper electrodes and their chemical environment opens a new chapter in the quest for effective CO2 reduction technologies. By refining our understanding and methodologies, we inch closer to viable solutions that can significantly impact our reliance on fossil fuels and contribute to environmental sustainability.