Polypropylene is an unsung hero in the world of materials, quietly pervading everyday products that range from food containers to medical devices. Its versatility and durability make it indispensable in various industries, reinforcing its status as a material of choice. However, with increased awareness about sustainability and environmental impact, the production process of polypropylene is under scrutiny. This calls attention to the underlying chemical processes that create propylene, the essential building block of polypropylene, and raises the stakes for innovation in this field.
The Quest for Efficiency in Propylene Production
At the heart of propylene production lies propylene itself, derived from propane, a natural gas commonly associated with outdoor barbecues and home heating. The traditional method of converting propane into propylene employs metal catalysts, typically chromium or platinum, which, although effective, come with significant energy costs and environmental drawbacks. These traditional catalysts operate at high temperatures, which not only escalates energy use but also contributes to increased carbon emissions—a pressing concern in the fight against climate change.
Recognizing the urgent need to make propylene production more efficient, researchers at the U.S. Department of Energy’s Argonne National Laboratory and Ames National Laboratory have begun to chart new territory. Their innovative research, recently published in the Journal of the American Chemical Society, introduces a promising avenue for faster and more energy-efficient methods for converting propane into propylene.
Unveiling the Power of Zirconium and Silicon Nitride
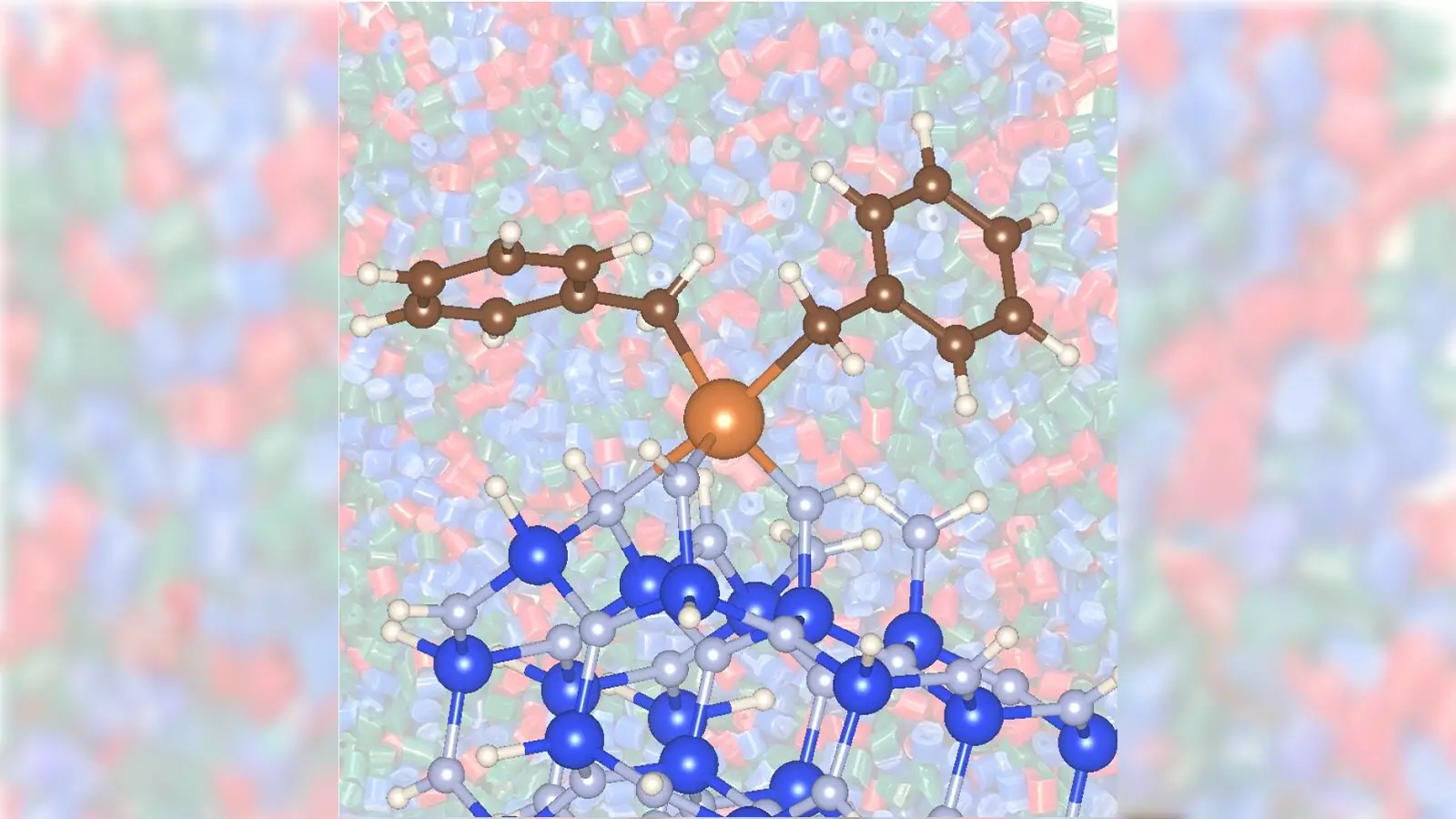
The groundbreaking element in this new process is the combination of zirconium and silicon nitride, which has shown an outstanding ability to enhance the ability to convert propane gas into propylene. This particular research suggests that using zirconium as a catalyst on silicon nitride support can dramatically speed up reaction rates while simultaneously reducing energy consumption and toxicity, a concern often associated with traditional catalysts.
Silicon nitride, less commonly used in catalytic processes, has emerged as a game-changing support material. Unlike traditional oxide supports, silicon nitride enhances chemical reactions on metal surfaces and has demonstrated faster catalytic performance, redefining the expected standards of efficiency in chemical reactions. The findings from the Argonne and Ames collaboration illuminate a pathway that not only minimizes energy use but also showcases how alternative materials can significantly impact catalysis.
Breaking Down the Numbers: A New Temperature Benchmark
One of the most critical revelations from this research is the drastic reduction in necessary operating temperatures. Traditionally, catalytic conversion occurs at about 1,022 degrees Fahrenheit, a benchmark that demands substantial energy input. However, the study revealed that the conversion process can occur effectively at just 842 degrees Fahrenheit with the new catalysis approach. This reduction is not merely incremental; it represents a significant leap that could effectively slash energy costs and carbon dioxide emissions associated with propylene production.
The implications of these lower temperatures are profound, especially considering that carbon dioxide is a major contributor to greenhouse gas emissions in the United States. By shifting the temperature requirements for catalysis downward, this innovation has the potential to contribute to a more sustainable chemical production landscape.
A Glimpse into Future Catalysis Trends
The potential applications of this research extend beyond the immediate goal of propylene production. The exploration into the reactivity achievable with low-cost metals presents exciting possibilities across various chemical processes. Researchers like David Kaphan and Max Delferro are keenly aware that the implications for other important chemical reactions are vast. The findings validate a fundamental approach that could be mirrored in other catalytic systems, potentially leading to more efficient processes in a range of industries.
Building on this momentum, the research team aims to delve deeper into the advantages provided by nitride-supported catalysts. With their expertise and innovative methodologies, they are poised to unlock further efficiencies in catalysis, paving the way for more sustainable industrial processes.
The Passion Behind the Research
At the core of this collaborative effort is a dynamic team of scientists who share a vision for sustainable chemistry. The interdisciplinary approach harnesses expertise from various domains, emphasizing that no single individual holds the key to breakthroughs like these. Collective efforts, as highlighted by contributors from Argonne and Ames, illustrate the importance of teamwork in tackling complex challenges.
The excitement within the research community, especially regarding the relatively unexplored surface compositions of silicon nitride, reflects a growing enthusiasm for innovative materials science. Such enthusiasm may well be the driving force behind future discoveries that could achieve even greater innovations in catalysis and sustainable chemical production.
In a world increasingly shaped by environmental considerations, the shift towards a more efficient and less toxic production of essential materials like propylene appears not just beneficial but necessary. With the unfolding of these promising initiatives, we stand on the threshold of a greener future—one driven by groundbreaking scientific inquiry and passion for innovation.